|
PAIN |
|
|
Laurance Johnston, Ph.D.
Sponsor: Institute of Spinal Cord Injury, Iceland |
| |
Prevalence of SCI Pain
SCI Pain Classification
Pharmaceutical Approaches:
Anticonvulsants (Gabapentin,
Pregabalin, Lamotrigine)
Antidepressants (Amtriptyline)
Analgesics (Lidocaine.
Ketamine, Alfentanil, Tramadol, Morphine, Clonidine, Capsaicin)
Antispasticity (Baclofen,
botulinum toxin, i.e., Botox, Cannabis or THC)
Surgical Approaches:
Dorsal Root Entry Zone Lesioning
Other Pain-Management Techniques:
Acupuncture
Hypnosis
Transcutaneous Electrical Nerve Stimulation (TENS)
Healing Touch
Vitamin D
Emotional Freedom Technique
Exercise 
SCI PAIN
MANAGEMENT
People with SCI often have some form of pain that
compromises quality of life and the ability to carry out many
activities. Pain can result from both damage to the spinal cord itself
and the lifestyle imposed by the neurological damage (e.g., wheelchair
transfers, etc). Unfortunately, efforts to manage such pain have been
challenging.

PREVALENCE OF SCI PAIN
Numerous studies document the high prevalence of
pain in individuals with SCI, including the following:
1) A survey of 380 individuals with SCI by Dr.
P. Fenollosa et al indicated that 66% had experienced chronic pain
lasting longer than six months. The most common type of pain was
deafferentation or phantom pain due to the loss of sensory input into
the central nervous system.
2) Dr. S. Stormer and colleagues (Germany)
reported that 66% of 901 surveyed patients with SCI had either pain or
pain-related sensations called dysesthesia (uncomfortable, abnormal
sensations such as burning, wetness, itching, electric shock, and pins
and needles). Sixty-one percent of them rated their pain intensity 7+ on
a scale ranging from 0 (no pain) to 10 (as bad as it can get).
Seventy-five percent described the pain “as rather or very distressing.”
In these patients, 86% reported that the pain was located below the
spinal-cord-injury lesion or in the transition zone surrounding it.
3) Dr. A. Ravenscroft and associates (United
Kingdom) sent a questionnaire to 216 individuals with SCI listed on a
regional SCI database. Of the 67% who responded, 79% indicated that they
currently suffered from pain, with 39% describing it as severe. The
survey results suggested that complete injury was more likely than
incomplete injury to result in chronic pain.
4) Dr. Nanna Finnerup and colleagues
(Denmark) mailed a questionnaire to 436 outpatients of a SCI
rehabilitation center, of whom 76% responded. The time since injury in
these individuals ranged from 0.5 to 39 (average 9.3) years. Overall,
77% of the respondents reported having pain or unpleasant sensations
(e.g., dysesthesia), including 67% reporting pain or unpleasant
sensations below the area of injury. Nearly half reported that
pain-related sensations could be triggered by stimulation of the skin by
non-noxious processes that do not normally provoke pain (a condition
called allodynia).
5) Dr. P. Siddall et al. (Australia)
followed the evolution of pain in 73 patients for five years after
injury. Eighty-one percent reported the presence of pain, the most
common form being musculoskeletal pain (59%), followed by at-level
neuropathic pain (41%), below-level neuropathic pain (34%), and visceral
pain (5%), respectively. [These different forms of SCI-related pain are
discussed below.]
6) Dr. D. Cardenas et al (USA) reviewed the
health records of 7,379 individuals with SCI, who had been entered in a
national SCI database. Data analyses indicated that the prevalence of
pain remained fairly constant over time, for example, 81% reporting pain
one year after injury and 83% 25 years after injury. Although no gender
difference was noted, pain prevalence was lower in nonwhites.
7) Dr. C. Donnelly and associates (Canada)
examined the records of 66 individuals with SCI who had been
consecutively admitted to a tertiary rehabilitation center. Six months
after discharge, 86% reported pain, with 27% reporting pain severe
enough to affect many or most activities.

SCI PAIN
CLASSIFICATION
There are many different forms of SCI-associated
pain. For example, the pain can be located above the level of
neurological injury, at or near the injury level, or below the injury
level. In addition, the pain can be either nociceptive or
neuropathic in origin. Nociceptive pain occurs from damage to
non-neural tissues, such as bones, connective tissue, muscle, skin, or
other organs, that are still partially or fully innervated. It can be
mechanical or musculoskeletal in nature, or arise from damage to or
irritation of internal visceral organs affected by SCI.
As the name implies, neuropathic pain results from
damage to neural tissue either within the peripheral (nerves outside of
the brain and spinal cord) or central nervous system. Two common forms
of SCI-neuropathic pain are central and radicular pain. The former is
caused by damage to the spinal cord itself. The latter is caused by
damage to nerve roots where they connect to the spinal column due to
damage from the initial injury or impingement by bone fragments or disk
or scar material.
Studies suggest that there are changes in the
properties of nerve cells close to the injury site, including 1)
increased responsiveness to peripheral stimulation, 2) more background
activity, and 3) extended neuron firing following stimulation. Overall,
injury results in altered neurotransmission and, as such, the firing
properties of spinal neurons.
As indicated in the table, Drs. Thomas Bryce and
Kristjan Ragnarsson (USA) have developed a SCI pain classification system that integrates these
concepts into 15 different types of pain.
have developed a SCI pain classification system that integrates these
concepts into 15 different types of pain.
For illustration sake, a number of these categories
are amplified below:
Type 1: An example
of above-level, nociceptive pain of mechanical or musculoskeletal origin
is shoulder pain resulting from transfers, rotator cuff injuries, etc.
Type 2: An example
of this sort of pain is a headache from autonomic dysreflexia (see
glossary).
Type 4:
Above-level, compressive neuropathic pain is generated from impingement
of a specific peripheral nerve, an example being carpel tunnel syndrome
resulting from the repetitive actions of wheelchair pushing.
Type 6: At-level
nociceptive pain of mechanical or musculoskeletal origin is similar to
Type 1 pain except located nearer the level of injury, e.g., shoulder
pain in the case of a cervical injury.
Type 7: At-level
nociceptive pain of visceral origin results from damage, irritation, or
distension of internal organs. An example is pain resulting from fecal
impaction or bowel obstruction.
Type 8: At-level
neuropathic, central pain is caused by damage to the spinal cord. In
thoracic injuries, central pain is often characterized by tightness,
pressure, or burning; and in cervical injuries by numbness, tingling,
heat, or cold. The formation of a fluid-filled syringomyelia cavity
within the spinal cord often causes central pain.
Type 9: At-level
neuropathic, radicular pain is caused by damage to nerve roots at their
connection to the spinal column. Such damage is often due to the initial
injury or impingement by bone fragments, disk material, or scar tissue.
Pain is often described as radiating, stabbing, shooting, or
electric-shock like.
Type 10: At-level
compressive, neuropathic pain is similar to Type 4 pain, except located
nearer to the injury site. An example would be repetitive-motion-created
carpal tunnel syndrome in an individual with a cervical injury.
Type 11: With
complex regional pain syndrome, pain is 1) not limited to the region
of a single peripheral nerve or nerve root, 2) out of proportion to what
is expected, and 3) associated with edema, skin blood-flow abnormality,
or irregular activity of the nerves that stimulate sweat glands (called
sudomotor activity). It is associated with diffuse hand pain, swelling
and stiffness.
Type 12:
Nociceptive mechanical or musculoskeletal pain below the level of injury
occurs in individuals with incomplete injuries or complete injuries with
a zone of partial preservation extending to the level of the pain. It is
often associated with spasticity.
Type 13:
Below-level, nociceptive visceral pain is primarily due to damage,
irritation, or distension of internal organs. It occurs in individuals
with injuries above the mid-thoracic region and is often vague and
poorly localized in nature.
Type 14:
Below-level, neuropathic central pain is caused by damage to the spinal
cord. It is often regional in nature affecting large areas such as the
anal region, the bladder, the genitals, the legs or even the entire body
below the injury level. The pain has been described as burning or aching
and often continuous in presence.

TREATMENTS FOR SCI-RELATED PAIN
Many approaches have been developed for treating
SCI-related pain, ranging from the pharmaceutical to the surgical to the
alternative. In general, these approaches have had modest success at
best, often depend upon the specific pain that is manifesting, and are
frequently accompanied by significant side effects. Overall, pain
management is a challenging problem, which will require the continued
effort of clinicians and researchers to develop effective solutions.
PHARMACEUTICAL APPROACHES
For better or worse, pharmaceutical approaches
remain the cornerstone of most SCI-pain-controlling strategies. Many
different drugs developed for a variety of purposes have been used in an
effort to ameliorate SCI pain, including anticonvulsants, analgesics,
antispastics, antidepressants, nonsteroidal anti-inflammatory drugs,
etc.

Anticonvulsant
Drugs
Anticonvulsants are a diverse group of drugs
developed for the treatment of epileptic seizures. They have been
adopted for use in treating SCI pain because scientists have noted a
similarity between the underlying physiology or biochemistry observed in
seizure disorders and neuropathic pain, both of which involve abnormal
firing of neurons. Several studies summarizing the use of a number of
anticonvulsant drugs to treat SCI pain are provided below:
1) Initially developed to treat
epileptic seizures, gabapentin has been used to manage
neuropathic pain after SCI. Structurally related to a key
neurotransmitter called GABA (gamma-amino butyric acid), evidence
suggests that gabapentin interferes with the transport of calcium ions
into neurons, a process involved in the excitation of neurons.
|
GABA |
Gabapentin |
|

|
|
A) Dr. Funda Levendoglu and associates
(Turkey) examined the effectiveness of gabapentin in ameliorating
neuropathic pain in 20 subjects (13 males, 7 females) with complete
paraplegia. The subjects ranged in age from 23 to 62 (mean 36) years,
and the time since injury varied from seven to 48 months.
The study was designed as a prospective,
randomized, double-blind, placebo-controlled, crossover clinical trial.
Basically, under this study design, an equal number of subjects were
randomized to receive either gabapentin or placebo in identical
capsules. In the first four weeks, the subjects received increasing
doses of the drug/placebo until the maximum dosing level was achieved,
which was maintained for four more weeks. After a two-week washout
period in which no drug or placebo was administered, treatments were
reversed for another four weeks; i.e., gabapentin-treated subjects now
received placebo and vice versa.
Pain was measured by several different scales,
including the Visual Analog Scale (VAS). With this scale, s ubjects
rated their pain levels on a scale ranging from 0 (no pain) to 100
(worst pain imaginable). As can be seen from the table, the pain levels
of gabapentin-treated subjects declined significantly over the treatment
period relative to placebo. ubjects
rated their pain levels on a scale ranging from 0 (no pain) to 100
(worst pain imaginable). As can be seen from the table, the pain levels
of gabapentin-treated subjects declined significantly over the treatment
period relative to placebo.
With the Neuropathic Pain Scale, subjects
rated their pain levels on a scale from 1 to 10 for different aspects of
neuropathic pain, including that described as sharp, hot, dull, cold,
sensitive, itchy, unpleasant, deep or surface pain. Any score above 4
was considered moderate to severe pain. Over time, gabapentin treatment
provided a statistically significant reduction in pain for all aspects
except for the itchy, dull, sensitive, and cold categories. For example,
before gabapentin treatment, the score for deep neuropathic pain
averaged 7.0, but after eight weeks of treatment, it averaged only 3.5.
Sixty-five percent and 25% of the gabapentin and
placebo-treated patients, respectively, reported various side effects,
such as nausea, vomiting, weakness, edema, vertigo, sedation, headache,
diarrhea, blurred vision, muscle twitching, and itching.
B) Dr. T.P. To et al (Australia)
retrospectively reviewed the health records of 38 individuals with SCI
to assess gabapentin’s potential to alleviate neuropathic pain. Age
averaged 47 (range 15 -75) years. There were 28 males; 19 and 16 with
parap legia
and 16 tetraplegia, respectively; and three times more chronic than
acute (< six months) injuries. The review indicated periodic assessments
of pain using the Visual Analog Scale, which, in this case, ranged from
0 (no pain) to 10 (worse pain imaginable). legia
and 16 tetraplegia, respectively; and three times more chronic than
acute (< six months) injuries. The review indicated periodic assessments
of pain using the Visual Analog Scale, which, in this case, ranged from
0 (no pain) to 10 (worse pain imaginable).
Using this scale, 29 of the 38 patients had some
degree of pain relief due to gabapentin. There were eight reports of
adverse effects, most notably drowsin ess.
Eleven patients had pain levels assessed at one, three, and six months
after starting gabapentin treatment. As indicated in the table, average
pain levels in these 11 patients decreased form 8.9 to 4.0 after six
months. ess.
Eleven patients had pain levels assessed at one, three, and six months
after starting gabapentin treatment. As indicated in the table, average
pain levels in these 11 patients decreased form 8.9 to 4.0 after six
months.
C) Dr. Sang-Ho Ahn and associates (Korea)
evaluated gabapentin’s effectiveness in treating neuropathic pain in 31
subjects with SCI. These individuals were divided into two groups: 1) 13
whose duration of pain was less than six months and 2) 18 whose duration
of pain had lasted more than six months. Subject age averaging 45-46
years old; Group 1 was composed of seven and six individuals with
tetraplegia and paraplegia, respectively; and Group 2 included six and
12 individuals with tetraplegia and paraplegia, respectively.
Before gabapentin treatment, all patients had been
treated with a variety of other pain medications without improvement.
While continuing these preexisting medication regimens, increasing
gabapentin doses were administered to the patients until a maintenance
dose was reached after 18 days. This maintenance dose was continued for
eight weeks. Pain was periodically measured using the aforementioned VAS
scale. In addition, interference of sleep by pain was assessed on a
scale ranging from 0 (no interference) to 10 (unable to sleep because of
pain).
Of the 31 subjects initially recruited, 25
completed the study. For both groups, the amount of pain and sleep
interference was significantly reduced after eight weeks of gabapentin
treatment. The reduction was greater in Group 1 (pain duration < 6
months) than Group 2 (pain duration > 6 months). Specifically, the
average pain score for Group 1 decreased from 7.3 to 3.0 by eight weeks,
whereas the Group 2 score decreased from 7.6 to 5.1. In the case of
sleep interference, the Group-1 average score decreased from 5.7 to 1.8,
while the Group-2 score declined from 5.9 to 4.2.
D) In an effort to determine gabapentin’s long-term
effectiveness, Dr. John Putzke and colleagues (USA) identified 31
patients who had been treated with the drug for up to three years. Of
these 31 patients, 76% were men, 67% had paraplegia, 76% had incomplete
injuries, and 86% reported pain at or below the level of their injury.
Twenty seven of the initial 31 identified patients
were contacted six months after initiating gabapentin treatment. Of
these 27, six had discontinued treatment due to intolerable side
effects. The remaining 21 rated their pain on a scale raging from 0 (no
pain) to 10 (most excruciating pain imaginable). Fourteen (67%) of these
21 patients reported a favorable reduction in pain over this six-month
time period defined as a 2+ point reduction on this 0-10 scale. Of these
14 subjects, 11 were contacted three years after initiating gabapentin
treatment. Ten of these 11 continued to report pain-relieving benefits
that they attributed to gabapentin. Side effects included fatigue,
forgetfulness, edema, gastrointestinal upsets, sedation, blurred vision,
dry mouth, constipation, and dizziness.

2) Pregabalin is also an anticonvulsant drug
specifically developed to treat neuropathic pain as well as epileptic
seizures. Like gabapentin, pregabalin is stru ctural
analog of the GABA neurotransmitter. It also apparently works by
affecting calcium ion influx into neurons, which, in turn, modulates the
firing of neurons involved in triggering pain sensations. ctural
analog of the GABA neurotransmitter. It also apparently works by
affecting calcium ion influx into neurons, which, in turn, modulates the
firing of neurons involved in triggering pain sensations.
A) Dr. Philip Siddall and colleagues
(Australia) evaluated pregablin’s effectiveness in treating central
neuropathic pain in subjects recruited from eight Australian centers. In
this study, 137 patients were randomized to receive either pregablin (70
patients) or placebo (67 patients) for 12 weeks. This was a double-blind
study, meaning neither patient nor physician knew who was receiving the
drug as opposed to the placebo. In the pregabalin-treated group, age
averaged 50 years; 60% were men; and 59% and 41% had paraplegic and
tetraplegic injuries, respectively. All subjects had been injured for at
least a year and had central neuropathic pain lasting three months
continuously or alternatively six months intermittently. Subjects were
allowed to continue preexisting pain-medication regimens (~70% of
subjects), except for gabapentin, which, due to its similarity to
pregablin, had to be discontinued a least week before starting the
study.
Starting the week before treatment (i.e., baseline
assessment) and throughout the 12 week treatment period, all subjects
rated their pain upon awakening in the morning for the preceding 24
hours on a scale from 0 (no pain) to 10 (worst possible pain). Using a
similar scale, they also rated the degree to which the pain interfered
with sleep.
The pain level in the pregablin-treated subjects
decreased from 6.5 before treatment to 4.2 at the end of the study. In
contrast, the pain levels for placebo-treated individuals only decreased
from 6.7 to 6.3. Forty-two percent of the pregablin-treated subjects had
at least a 30% reduction in pain compared to only 16% for the
placebo-treated individuals. In addition, 22% of the pregablin-treated
subjects had at least a 50% reduction in pain compared to only 8% for
those who were treated with placebo.
Furthermore, pregablin-treated patients had a
similar reduction in sleep problems. For example, in contrast to the
placebo-treated subjects who had only a minimal reduction in sleep
interference over the treatment period (4.9 to 4.7), the sleep
interference score decreased from 4.2 to 2.8 in pregablin-treated
subjects.
The most frequently reported adverse effects were
drowsiness (41%), dizziness (24%), edema (20%), weakness (16 %), dry
mouth (16%), and constipation (13%).
B) Dr. Jan Vranken and associates (The
Netherlands) examined pregabalin’s effectiveness in a randomized,
double-blind, placebo-controlled clinical trial. The investigators
recruited 40 subjects with a variety of neurological disorders
predisposing them central neuropathic pain, including 21 with complete
and incomplete spinal cord injuries. These individuals were randomized
to receive either pregabalin or placebo daily for four weeks. In
addition, they were allowed to continue any preexisting pain-medication
regimens if it had been stable in nature. The exception was gabapentin,
which had to be discontinued at least three days before study
initiation. To be enrolled, all subjects had to have a pain level of at
least 6 using the previously described 0-10 pain-intensity scale.
As shown in the graph, pain intensity was
significantly less in those treated with pregablin. Specifically,
although pain levels in placebo-treated individuals essentially remain ed
unchanged over the four-week trial period, pain in the pregabalin-treated
subjects decreased from 7.6 to 5.1, a decrease the investigators
described as a reduction from severe to modest. Seven pregabalin-treated
subjects had a reduction in pain of more than 50% compared with only one
placebo-treated subject. Roughly equal adverse effects were observed for
both the pregabalin and placebo group, indicating that, at least in the
case of this study, pregabalin-related side effects were minimal. ed
unchanged over the four-week trial period, pain in the pregabalin-treated
subjects decreased from 7.6 to 5.1, a decrease the investigators
described as a reduction from severe to modest. Seven pregabalin-treated
subjects had a reduction in pain of more than 50% compared with only one
placebo-treated subject. Roughly equal adverse effects were observed for
both the pregabalin and placebo group, indicating that, at least in the
case of this study, pregabalin-related side effects were minimal.

3) Lamotrigine is another anticonvulsant
drug used to treat epilepsy, bipolar
 disorder,
and, secondarily, neuropathic pain. Unlike gabapentin and pregabalin, it
is not a structural analog of the GABA neurotransmitter. disorder,
and, secondarily, neuropathic pain. Unlike gabapentin and pregabalin, it
is not a structural analog of the GABA neurotransmitter.
Dr. Nanna Finnerup and associates (Denmark)
evaluated lamotrigine’s pain-treating effectiveness in 30 individuals
with SCI-related neuropathic pain below the level of the lesion. To be
enrolled, subjects had to have a 3+ pain level on the 0 (no pain) to 10
(worst imaginable pain) scale discussed previously. The study was
designed as a randomized, double-blind, placebo-controlled, crossover
trial. Specifically, subjects were randomized to receive either
lamotrigine or placebo for nine weeks, after which there was a two-week
washout period in which no drug/placebo was given. When this washout
period was finished, treatments were reversed and the lamotrigine-treated
subjects now received placebo for nine weeks, and the placebo-treated
individuals were given the active drug.
Of the 30 enrolled patients, 22 completed the
study. Of these remaining subjects, age ranged from 27 to 63 (average
49) years; 18 were men; and 9, 11 and 2 had cervical, thoracic, and
lumbosacral injuries, respectively (including both complete and
incomplete injuries). Study results indicated that lamotrigine only
reduced pain levels in those with incomplete injuries. Specifically, for
these individuals, the difference in pain reduction between drug- and
placebo-treated averaged a modest 25%. The drug had had no statistical
significant effects for those with complete injuries. The number of
adverse side effects were comparable in both lamotrigine and placebo
groups.

Antidepressants
Drugs
Several antidepressant drugs have been used to
treat SCI pain, including the following:
1) Amitriptyline treats depression symptoms
by raising the levels of naturally occurring substances in the central
nervous system. For example, like other antidepressants, amitriptyline
increases serotonin, a key mood-influencing neurotransmitter. In
addition to depression, the drug has been used to treat pain generated
from a variety of disorders, including SCI.
In a 2007 article, Dr. Diana Rintala and
colleagues (USA) compared the effectiveness of amitriptyline relative to
gabapentin in ameliorating chronic, SCI-associated neuropathic pain.
Thirty-eight individuals with SCI were randomized to receive either
amitriptyline, gabapentin, or an active placebo (Benadryl, an
over-the-counter allergy medication). After a baseline interval in which
subjects received no pain medications, one of these three agents was
administered for nine weeks. This was followed by a one-week washout
period in which no drugs were administered. Thereafter, a different drug
was administered for another nine-week period, e.g., the
amitriptyline-treated individuals were now given gabapentin or placebo,
etc. After another washout period designed to remove residues from the
body of the previously administered drug, the third agent would be
given, e.g., the subjects who had been initially given amitriptyline
followed by gabapentin were now treated with placebo, etc.
Of the 38 subjects who started the study, 22
completed the study. Average age was 43 (range 22-65) years; time since
injury averaged 13 (range 1-33) years; and 90% were men. Subjects
included individuals with both tetraplegia and paraplegia, as well as
complete and incomplete injuries.
Pain was periodically assessed using the previously
discussed VAS measure which subjectively rated pain on scale ranging
from 0 (no pain) to 10 (worst possible pain). In addition, depression
levels in subjects were periodically evaluated using another subjective
scale.
The overall results indicated that amitriptyline
was more effective than gabapentin in relieving pain. Specifically,
after eight weeks of treatment, pain levels on the 0-10 VAS scale
averaged 3.5 for amitriptyline-treated subjects, 4.8 for
gabapentin-treated subjects, and 5.1 for placebo-treated subjects.
Underscoring the relationship of pain to depression, amitriptyline’s
pain-relieving benefits were greater in those individuals who started
the study with the most depression. Documented side effects for
amitriptyline included mouth dryness, constipation, increased
spasticity, and painful urination.
Different results were observed in an earlier study
(2002) carried out by Dr. Diana Cardenas and colleagues (USA). In
this study, 44 and 40 subjects were randomized to receive either
escalating doses of amitriptyline or placebo, respectively, for six
weeks. Because a common side effect of amitriptyline is dry mouth, an
active placebo (i.e., not inert) was chosen that also produced dry mouth
(specifically, benztropine, a drug used for Parkinson’s disease). This
was done to preserve the study’s blinded nature so that subjects could
not readily distinguish amitriptyline from the placebo.
In the amitriptyline-treated subjects, age ranged
from 21 to 63 (average 41) years; 59%, 39%, and 2% had cervical,
thoracic, and lumbar/sacral injuries, respectively; approximately half
had complete injuries; and 73% were men. The average time since injury
was about 13 years.
As in the previously discussed studies, pain was
periodically evaluated on a scale ranging from 0 (no pain) to 10 (as bad
as could be). In this study, no statistically significant difference in
pain levels was observed between the amitriptyline and placebo-treated
groups. One possible reason for the different outcomes compared to the
previous discussed study is that the Rintala study was limited to
individuals with neuropathic pain while the Cardenas study included a
variety of types of SCI-related pain, each of which may respond
differently to various medications.

Analgesics
A number of traditional painkilling drugs have
demonstrated some effectiveness in treating SCI-related pain, including
the following:
1) Lidocaine has a variety of medical
applications, pain killing and otherwise. Most commonly it has been used
as a topical agent to relieve itching, burning, and pain from skin
inflammation; or through injection as a dental numbing agent or as a
local anesthetic for minor surgery. In addition, it has been
intravenously administered to treat abnormal heart rhythms, i.e.,
arrhythmias. Physiologically, lidocaine affects the flux of sodium ions
into neurons needed to propagate nerve signals. By so doing, scientists
theorize that the neuronal hyperexcitability that characterizes SCI
neuropathic pain may be dampened.
A) In 2005, Dr. Nanna Finnerup et al
(Denmark) reported the results of a study treating 24 subjects with
neuropathic pain at or below the level of the injury with lidocaine.
Subjects were randomized to receive either an intravenous infusion of
lidocaine or saline solution. Subject age ranged from 28 to 66 years; 17
were men; 9, 12, and 3 had cervical, thoracic, and lumbosacral
injuries/dysfunction, respectively; and the sample included a range of
both complete and incomplete injuries. Among other measures, pain was
assessed on a subjective 0-100 scale before infusion, and 25 and 35
minutes after infusion was started. After at least six days, treatments
were reversed; i.e., lidocaine-treated subjects now received the placebo
infusion and vice versa.
The average difference in pain reduction between
lidocaine- and placebo-treated subjects was 36%. Eleven
lidocaine-treated subjects had at least a 33% reduction in pain compared
to only two placebo-treated subjects. Nineteen lidocaine-treated
subjects experienced various adverse side effects, including drowsiness,
dizziness, impaired speech, lightheadedness, blurred vision, etc.
B) In 2000, Dr. N. Attal and associates
(France) evaluated the effectiveness of intravenously administered
lidocaine in alleviating pain in 16 individuals with stroke (6) or
spinal cord injury/dysfunction (10). The study focused on central pain,
including spontaneous ongoing pain and evoked pain such as allodynia
produced by stimuli that does not normally provoke pain (such as skin
brushing; see introductory discussion. Of the 16 patients enrolled, 10
were women and six men; mean age was 55; and duration of pain averaged
47 months. Subjects were randomized to receive either a 30-minute,
intravenous infusion of lidocaine or saline solution. Among other
measures, pain levels were assessed before treatment and periodically
thereafter using a subjective pain scale ranging from 0 (no pain) to 100
(worst possible pain).
When compared to controls, the lidocaine-treated
subjects had statistically significant less spontaneous pain at the end
of the treatment and for up to 45 minutes afterwards. Specifically, the
pain levels in lidocaine-treated subjects decreased from 61 to 31 while
the pain levels in placebo-treated subjects decreased only to 46.
However, after 45 minutes, the difference in pain levels between the two
groups diminished. Similarly, lidocaine-treatment reduced the intensity
of allodynia for 30 minutes after treatment was completed. Few, if any,
long-term benefits were observed. The investigators concluded that “in
least in patients with central pain, long-term analgesic effects of
lidocaine are uncommon.” Adverse side effects included
lightheadedness/dizziness, drowsiness, nausea/vomiting, impaired speech,
malaise, etc.
C) In 1991, Dr. P. G. Loubser and associates
(USA) evaluated lidocaine’s pain-killing effectiveness in 21 individuals
with chronic SCI. In this study, subjects were randomized to receive
either lidocaine or saline placebo by injection into the lumbar
subarachnoid space (i.e., the area filled with cerebrospinal fluid).
After a sufficient washout period, treatments were reversed. Subject age
ranged from 18 to 58 (average 42) years; 14 subjects were men; and 5,
14, and 2 had cervical, thoracic, and lumbar injuries, respectively. All
subjects had had chronic pain of at least six months duration.
Pain was assessed before and periodically after
treatment using a variety of assessments. Thirteen lidocaine-treated
subjects showed an average 38% reduction in pain lasting on average
about two hours. Eight lidocaine-treated subjects showed no changes. In
a many subjects, lidocaine affected the distribution of pain throughout
the body and nature of the pain sensations. In a number of cases, there
were spinal canal blockages, which prevented the
lumbar-region-administered lidocaine from reaching and exerting
painkilling effects in areas above the blockage.
2) Ketamine has been primarily used to
generate brief periods of anesthesia, during which the patient feels
dissociated or separated from the body. Due to these
altered-consciousness effects, the drug has a history of substance
abuse. Ketamine has also been medically used to treat pain, depression,
and asthmatics or individuals with chronic obstructive airway disease.
Ketamine interferes with a key neurotransmission process involved in
generating pain.
In 2004, Dr. Ann Kvarnstrom et al (Sweden)
evaluated the effectiveness of intravenous ketamine and lidocaine in
treating below-level, neuropathic pain. Ten individuals with SCI were
randomized to receive 40-minute intravenous infusions of either
ketamine, lidocaine, or saline solution. After at least four days, one
of the other agents was similarly administered, and after another four
days, the final agent was given. Of the 10 subjects, nine were men; age
ranged from 30-60 (average 45) years; 1 and 9 had complete and
incomplete injuries, respectively; and 5, 4, and 1 had cervical,
thoracic, and lumbar injuries, respectively. The average pain duration
in subjects had been nine years.
Pain was evaluated before the start of the infusion
and 15, 45, 60, 120 and 150 minutes afterwards using the subjective 0
(no pain) to 10 (worst pain imaginable) scale. Using this scale, the
average pain reduction was 38% for the ketamine-treated subjects, 10%
for the lidocaine-treated subjects, and 3% for the placebo-treated
subjects. Five of the ketamine-treated subjects had at least a 50%
reduction pain compared to only one lidocaine-treated subject, and none
for placebo-treated subjects. Of the responders, all claimed that
ketamine was better than any other painkilling medications they had
tried.
Adverse side effects were common in both the
ketamine- and lidocaine-treated subjects, including drowsiness,
dizziness, out-of-body sensations, changes in hearing and vision,
nausea, etc.
3) Alfentanil is a potent, short-acting
opioid-like agent used for surgical anesthesia.
Opioids are psychoactive, naturally
occurring and synthetic molecules that bind to various receptors on the
surface of neurons, including those in the spinal cord. This binding
alters communication between neurons, which can mute pain perception.
The most well-known example of a naturally occurring opioid-containing
material is opium isolated from the poppy. Opium is the source of many
painkilling and substance-abuse drugs, such as morphine, its derivative
heroin, codeine. In addition, a number of opioid-like molecules are
actually produced by the body, such as the endorphins – a word actually
created by combining morphine and endogenous. Endorphins are
neurotransmitters associated with the feel-good endorphin rush or
runner’s high generated by exercise and other stimulus.
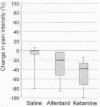
In 1995, Dr. Per Kristian Eide and
associates (Norway) compared the potential of both alfentanil and
ketamine to reduce pain after SCI. Nine patients were randomized to
receive an intravenous infusion of either alfentanil, ketamine, or a
saline placebo solution. Each drug infusion was separated by two hours.
Age ranged from 25-72 (average 41) years, and all but one of the
subjects were men. The sample included four cervical, four thoracic, and
one lumbar injuries, and five complete and four incomplete injuries. The
duration of pain in these subjects ranged from 14 to 94 months, starting
in all cases less than a half year after injury.
Continuous pain and pain evoked by various stimuli
was assessed using a VAS scale ranging from 0 (no pain) to 100
(unbearable pain). As shown in the graphs below, both alfentanil and
ketamine reduced both types of pain. Although no severe side effects
were observed, a variety of weak or modest side effects were noted for
both drugs, including nausea, fatigue, dizziness, mood changes, changes
in vision and hearing, feelings of unreality.
|
Change in Continuous Pain |
Change in Evoked Pain |
|
 |
 |
4) Another opioid drug, tramadol has been
extensively used to treat moderate to severe pain. In addition to
binding to neuronal opioid receptors, tramadol also increases levels of
serotonin, a key mood-influencing neurotransmitter.
In a 2009 study, Dr. Cecilia Norrbrink and
colleagues (Sweden) examined tramadol’s ability to relieve SCI-related
neuropathic pain. Of the 35 recruited subjects, 23 were randomized to
receive tramadol and 12 randomized to receive an identical appearing
placebo agent for an average of 21 days. Twenty-eight of the 35
recruited subjects were men; 16 and 19 had tetraplegia and paraplegia,
respectively; and the time since injury averaged 15 years. To avoid
biasing results, subjects maintained their existing pain-relieving
medications throughout the study.
Pain was evaluated using a variety of assessments,
including a 0-10 pain scale which combined numerical and verbal ratings.
Using this scale, subjects would periodically record various aspects of
the pain they had experienced, including intensity of present pain,
general pain, and worst pain. Compared to placebo, tramadol-treated
subjects had statistically significant less pain in all three of these
categories. In addition, tramadol-treated subjects had less anxiety and
greater life satisfaction and sleep quality.
Unfortunately, there was a high incidence of
adverse effects. Specifically, 21 of the tramadol-treated subjects (91%)
experienced at least one adverse effect, including 11 subjects that
withdrew from the study as a result. The most commonly reported adverse
effects were tiredness, dry mouth, and dizziness.
5) The most abundant opioid in opium, morphine
is used to treat severe pain. Due to its euphoria-producing,
anti-anxiety properties, the drug has considerable addictive and
substance-abuse potential. Morphine is closely related to heroin; in
fact, the body converts heroin to morphine before it binds to CNS
neurons. This binding produces the drug’s painkilling and psychoactive
effects.
In a 2002 study, Dr. N. Attal and colleagues
(France) examined the potential of intravenously administered morphine
to relieve central neuropathic pain in six patients with stroke and nine
with SCI. The study included nine women and six men with an average age
of 54 years. All subjects had had continuous pain of duration ranging
from 1.5 to 20 years. In this double-blind, placebo-controlled,
crossover study, subjects were randomized to receive either an
intravenous infusion of morphine or saline solution. Two weeks later,
the treatments were reversed, i.e., the morphine-treated subjects now
received the placebo infusion and vice versa.
Using a scale rating pain from 0 (no pain) to 100
(worst possible pain), pain intensity was assessed before treatment and
15, 30, 45, 60, 90, and 120 minutes afterwards. A variety of
central-pain components were assessed, including ongoing pain and pain
produced by stroking the skin with a brush (i.e., allodynia). With
respect to ongoing pain, seven subjects responded to morphine. However,
statistically there were no significant differences in pain levels
between the morphine- and placebo-treated subjects at any point in time.
In contrast, morphine produced a statistically significant reduction in
the brush-induced pain lasting up to 90 minutes after treatment. In nine
subjects, this evoked pain was reduced by at least 50% by the end of the
injection. The investigators concluded that morphine’s painkilling
benefits were probably limited to certain components of central pain.
The most frequent morphine-induced side effects were drowsiness, nausea,
and headaches.
Within one week of completing the study’s
intravenous phase, subjects began taking sustained-release, oral
morphine and started recording their pain levels daily using the
aforementioned 1-100 scale. Many of the subjects eventually dropped out
of the study due to unacceptable side effects of the oral morphine or
the absence of pain-relieving benefits. As a result, only three subjects
were still taking the oral morphine a year later. The investigators
noted that morphine-responsive subjects in the study’s intravenous phase
study were more likely to accrue benefits from oral morphine.
6) Clonidine has been used to treat high
blood pressure, various pain conditions, attention-deficit hyperactivity
disorder (ADHD), and anxiety/panic disorders.
In a 2000 study, Dr. Philip Siddall et al
(Australia) examined the potential of clonidine, morphine, and a
combination of the two to alleviate SCI-related neuropathic pain in 15
subjects with SCI. Ranging from 26 to 78 (average 50) years old,
subjects had below-level and/or at-level neuropathic pain (see
introductory discussion). In this study, the drugs were administered
into the lumbar-region, intrathecal space surrounding the spinal cord.
Subjects were randomized to receive clonidine, morphine, or saline via
this route of administration. When either a pain-relief or side-effect
response was observed, testing of the next drug was initiated the
following day. After all three agents had been tested, subjects received
the clonidine-morphine combination. Pain was assessed using a 0-100
rating scale and verbal pain rating (none, mild, moderate, severe, or
very severe).
Neither intrathecal administration of clonidine or
morphine resulted in a statistically significant reduction in pain.
However, intrathecal administration of the clonidine-morphine mixture
did result in statistically significant reduction. Specifically, the
drug combination resulted in an average reduction of pain to 63% of the
baseline score. A greater percentage of subjects with at-level,
neuropathic pain obtained substantial pain relief than those with
below-level neuropathic pain. The investigators suggested that this
difference may be due to the different physiological origins that
underlie at-level versus below-level neuropathic pain. The investigators
also noted that scarring around the injury site may inhibit the
migration of the drugs, which were intrathecally administered below the
injury site, to cervical regions above the injury site. Given the
relatively small sample size, this issue may have lessened observed
pain-reduction effects.
The most common side effects were itching (morphine
associated), low blood pressure (mostly clonidine associated), nausea,
sedation, and hypoxia (decreased oxygen levels).
7) Capsaicin is the active component of hot
peppers; it produces the hot sensation when the peppers are eaten.
Medicinally, it is used in topical ointments to relieve various types of
pain, e.g., backache, muscle sprains, etc. Physiologically,
capsaicin-exposed neurons are depleted of a key neurotransmitter (called
substance P) involved in transmitting pain signals. Basically, a
sustained capsaicin burning sensation overwhelms the neuron’s capability
to report pain, leading to a reduction in pain sensitivity.
In 2000, Drs. Paul Sanford and Paula Benes
(USA) reported the results of treating eight individuals with localized
pain at or just below the level of injury with capsaicin cream topically
applied four times daily (9). Age ranging from 18 to 66, six subjects
were men. All but one subject had paraplegia, and subjects were equally
divided between those complete and incomplete injuries. Patients who had
not responded to capsaicin (~ half of treated patients) were not among
the subjects included in this discussion – i.e., the article only
reported the positive results.
Subjects subjectively assessed their pain levels
using a 0 (no pain) to 10 (unbearable pain) scale. As shown in the
table, capsaicin-treated patients often had substa ntial
reductions in pain levels (again, only patients who benefitted are
reported). In most cases, pain levels increased again after capsaicin
treatment was discontinued. Other than initial burning sensations when
the cream was applied, few side effects were observed. ntial
reductions in pain levels (again, only patients who benefitted are
reported). In most cases, pain levels increased again after capsaicin
treatment was discontinued. Other than initial burning sensations when
the cream was applied, few side effects were observed.

Anti-Spasticity
Drugs
1) Baclofen is primarily used to treat
spasticity associated with various neurological disorders, including
SCI, multiple sclerosis, and cerebral palsy. Like several of the anti-convulsant
drugs previously discussed, baclofen is structurally related to GABA, a
key neurotransmitter involved in pain perception. Baclofen is given
either orally or infused into the intrathecal space surrounding the
spinal cord.
|
GABA |
Baclofen |
|

|
 |
A) Because chronic pain and spasticity often
co-exist, in 1992, Dr. Richard Herman and colleagues (USA)
evaluated baclofen’s ability to ameliorate pain in nine individuals with
spinal lesions due to SCI, MS, and transverse myelitis (disorder
involving inflammation of the spinal cord) (1). Three of the subjects
were men, and age ranged from 33 to 63. In a double-blind trial, seven
of the nine were randomized to receive on successive days either an
intrathecal infusion of baclofen or placebo. Baclofen treatment
significantly reduced dysesthetic pain (see earlier discussion) in six
of the seven randomized patients within 5-20 minutes of treatment. It
also eliminated all spasm-related pain. After this double-blind trial
had been discontinued, two additional individuals with SCI were treated
with baclofen. In one, dysesthetic pain was eliminated completely, and
in the other, spasm-related pain was markedly reduced. As the baclofen
cleared from the body, the pain returned 8-12 hours later.
B) In 1996, Drs. Paul Loubser and Nafiz
Akman (USA) reported the pain-reducing influence of baclofen
treatment intrathecally administered through an implanted pump (2).
Twelve treated patients had chronic pain before the intervention,
including six with neurogenic pain, three with musculoskeletal pain, and
three with both types of pain. All were men except one, age ranged from
21-63, and injury level was equally divided between cervical and
thoracic injuries. Pain status was evaluated before pump implantation
and 6 and 12 months afterwards using a variety of assessments, including
the previously discussed visual analog scale rating pain from 0 to 10.
Although no statistically significant reduction in
neurogenic pain was observed at either 6 or 12 months, five of the six
patients with musculoskeletal pain had a significant pain reduction. The
investigators concluded that “intrathecal baclofen reduces chronic pain
associated with spasticity but does not decrease neurogenic pain
symptoms when used at dosages aimed at controlling spasticity.”
2) The most powerful neurotoxin known, botulinum
toxin is produced by the bacteria Clostridium botulinum. At
one time, the fatality rate for botulinum poisoning was 60% due to
respiratory muscle paralysis. In spite of its lethality, botulinum toxin
has a variety of low-dose medical uses related to its ability to
decrease muscle activity. By far, its most well know application is
cosmetic (i.e., Botox injections) to prevent the development of wrinkles
through paralyzing facial muscles.
Due to its muscle-weakening ability, botulinum
toxin is also used to treat spasticity-associated hyperactive muscles
and dystonia-related involuntary muscle contractions. Botulinum toxin
prevents the release of the acetylcholine neurotransmitter from a neuron into the gap between the neuron and muscle. Under normal
circumstances, the released acetylcholine would interact with
muscle-cell receptors on the other side of the gap, activating the
muscle. In addition, evidence indicates botulinum toxin has the ability
to lessen pain distinct from its spasticity-lowering effects.
Specifically, botulinum toxin also appears to inhibit the release of
substance P, a neurotransmitter, which, as discussed previously for
capsaicin, is involved in transmitting pain signals.
a neuron into the gap between the neuron and muscle. Under normal
circumstances, the released acetylcholine would interact with
muscle-cell receptors on the other side of the gap, activating the
muscle. In addition, evidence indicates botulinum toxin has the ability
to lessen pain distinct from its spasticity-lowering effects.
Specifically, botulinum toxin also appears to inhibit the release of
substance P, a neurotransmitter, which, as discussed previously for
capsaicin, is involved in transmitting pain signals.
A) In 2008, Dr. C. Marciniak and associates
(USA) evaluated the use of botulinum toxin to treat spasticity and, as
one of several secondary assessments, reduce pain (3). In this
retrospective study, the charts of 28 individuals with SCI who had been
treated with botulinum toxin for spasticity were reviewed. Patient age
averaged 48 (range 20-76) years, and in 20, the cause of SCI was
traumatic injury. Of the six individuals who had identified pain as an
issue before treatment, five (83%) reported less pain afterwards. The
investigators did not know whether this pain reduction was the result of
less spasticity or due to botulinum toxin’s influence on pain
transmitters, such as substance P.
3)
Tetrahydrocannabinol (THC) is the active agent in cannabis, i.e.,
marijuana. Cannabis preparations have a long history of use for treating
various neurological disorders and pain, including being used thousands
of years ago as traditional Chinese and Indian (i.e., Ayurvedic) herbal
remedies.
 THC
binds to receptors on the surface of central-nervous-system cells.
Research suggests that this binding affects the activity of GABA, which,
as discussed before, is a key neurotransmitter involved in pain
perception. THC
binds to receptors on the surface of central-nervous-system cells.
Research suggests that this binding affects the activity of GABA, which,
as discussed before, is a key neurotransmitter involved in pain
perception.
A) In a 2007 study, Dr. U. Hagenbach
and colleagues (Switzerland) examined THC’s influence on primarily SCI
spasticity (4). In addition, a number of secondary effects were also
evaluated, including pain through self assessments.
 Twenty-five
subjects with SCI were initially recruited for the various study arms.
Of these, 11 and 14 had paraplegia and tetraplegia, respectively; all
but two were men; and age ranged from 19 to 73. Of the 22 subjects
treated with an oral THC preparation, 15 consumed the drug for six
weeks. Although the results indicated an initial statistically
significant reduction in pain, the effect did not persist over time. Twenty-five
subjects with SCI were initially recruited for the various study arms.
Of these, 11 and 14 had paraplegia and tetraplegia, respectively; all
but two were men; and age ranged from 19 to 73. Of the 22 subjects
treated with an oral THC preparation, 15 consumed the drug for six
weeks. Although the results indicated an initial statistically
significant reduction in pain, the effect did not persist over time.

SURGICAL APPROACHES
Dorsal Root
Entry Zone Lesions
Spinal nerves project off the left and
right side of the spinal cord to every part of the body through openings
in the vertebral column. As shown below, these spinal nerves are
composed of dorsal roots, which carry sensory information into the
spinal c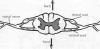 ord,
and ventral roots, which carry motor or movement information out of the
spinal cord toward muscles. The location where the dorsal roots enter
the spinal cord is called the dorsal root entry zone (DREZ). ord,
and ventral roots, which carry motor or movement information out of the
spinal cord toward muscles. The location where the dorsal roots enter
the spinal cord is called the dorsal root entry zone (DREZ).
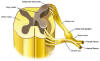 Although
specific mechanisms are still unclear, evidence suggests that the DREZ
is a key area in transmitting or processing pain stimuli. Spinal cord
injury or related neurological trauma, such as brachial plexus
nerve-root avulsion (nerve roots stretched or torn away from the cord,)
triggers aberrant activity within this pain-processing area. As such,
surgical procedures were developed to destroy the DREZ tissue producing
this dysfunctional activity. Although
specific mechanisms are still unclear, evidence suggests that the DREZ
is a key area in transmitting or processing pain stimuli. Spinal cord
injury or related neurological trauma, such as brachial plexus
nerve-root avulsion (nerve roots stretched or torn away from the cord,)
triggers aberrant activity within this pain-processing area. As such,
surgical procedures were developed to destroy the DREZ tissue producing
this dysfunctional activity.
 Basically
with these procedures, after the spinal cord is exposed through a
laminectomy, radiofrequency, laser, or other devices are used to produce
a series of lesions in the DREZ tissue in the problematic area of the
spinal cord. Basically
with these procedures, after the spinal cord is exposed through a
laminectomy, radiofrequency, laser, or other devices are used to produce
a series of lesions in the DREZ tissue in the problematic area of the
spinal cord.
Although these DREZ-lesioning procedures
have had some success in reducing certain types of SCI-associated pain,
they are not innocuous. Numerous complications have been observed,
including, because nervous tissue is being destroyed in an inexact
process, the further loss of sensation and function. As more
function-restoring strategies have emerged in recent years, the tradeoff
between not-guaranteed pain reduction versus the potential loss of
additional function combined with the inability to access these emergent
strategies has become more questionable.
The following summarizes key studies
evaluating the pain-reducing effectiveness of DREZ lesioning.
1) The radiofrequency DREZ-lesioning
procedure was initially described by Drs. Blaine Nashold and Roger
Ostdahl (USA) in 1979 for pain caused by nerve-root avulsion. Using
an electrode with a two-millimeter tip, about 10-20
radiofrequency-coagulation lesions spaced at 2-3-millimeter intervals
were made in the DREZ area associated with the avulsed roots. These
lesions gradually coalesced to produce a coagulation strip extending
from the uppermost to lowermost intact root. Of the 18 patients with
intractable pain due to brachial plexus avulsion injuries, 13
experienced good, lasting pain relief (i.e., 75+% pain relief). However,
a number experienced mild to moderate lower extremity weakness on the
same-side after the procedure.
2) Building upon their success described
previously for brachial plexus avulsion injuries, in 1980, Drs.
Blaine Nashold and Elizabeth Bullitt (USA) reported the results of
using DREZ lesioning to reducing central pain in 13 individuals with
lower-level spinal cord injuries. These injuries involved damage to the
conus medullaris (the terminal end of the spinal cord) or cauda equina
(a bundle of nerves occupying the vertebral column below the spinal
cord). All subjects suffered from lower extremity pain starting several
days to 12 years after injury. With age ranging from 35-60 years, five
subjects were females and seven were males.
Using DREZ-lesioning procedures similar
to those previously described, between seven and 16 lesions were made on
each side of the cord, extending from one or two levels above the injury
level down to the injury level or slightly below. Patients were followed
from 5-38 months. All but two reported at least a 50% reduction in pain,
with seven completely free of pain. Most patients were able to reduce
their pain medications. Unfortunately, three patients lost significant
function afterwards.
3) In 1984, Dr. Hans-Peter Richter and
Klaus Seitz (Germany) reported the results of using DREZ-lesioning
procedures to treat neuropathic pain in 10 patients (9 males, 1 female).
Of these patients, eight had cervical injuries, mostly due to accidents,
resulting in nerve-root avulsion, and two had thoracic injuries. Age
ranged from 17-68 years. The DREZ-lesioning procedures were similar to
those described above. Of the patients with cervical injuries, one died
six days and another 36 days after surgery. The surviving patients were
followed for 5-30 months. Of these, three were entirely pain free, one
had residual pain, and two had no reduction in pain levels. In both
patients with thoracic injuries, pain levels were the same as before the
surgery.
4) Also in 1984, Drs. Madjid Samii
and Jean Richard Moringlane (Germany) reported the results of
treating 35 patients with DRES lesioning, including 22 with brachial
plexus avulsion injuries and five with spinal cord injuries. Age ranged
from 18-59 years; and 28 patients were male, and seven were female. The
time period between pain onset and the DREZ-lesioning procedure ranged
from three months to 35 years. Of the 22 patients with brachial plexus
avulsion injuries, 17 had very good pain relief (defined as >70%
reduction in pain), three had good pain relief (50-70% reduction in
pain), and two had fair pain relief (<50% reduction in pain). Of the
five patients with SCI, two, two, and one had very good, good, and fair
pain relief, respectively. After the procedure, 18 patients suffered
from transient sensory and/or motor deficits.
5) Due to the complications associated
with radiofrequency DREZ-lesioning, Dr. Stephen Powers and
associates (USA) used microsurgical lasers to produce more precise,
smaller, and reproducible lesions. Of the 21 patients, seven with age
ranging from 27-48 years had pain associated with paraplegia. Followed
for periods ranging from 2-19 months, six of these seven patients with
SCI reported greater than 50% pain relief. In the overall treatment
group of 21 patients, transient and persistent sensory abnormalities
were observed in seven individuals.
6) In 1986, Dr. Allan Friedman and
colleagues (USA) summarized the results of using radiofrequency
DREZ-lesioning to treat intractable pain in 56 individuals with SCI.
Patient age ranged from 27-72 years, and all had suffered pain for at
least eight months before surgery. Pain relief was considered 1) “good”
if the patient was either free of pain or the pain did not require
analgesics or compromise daily activities, 2) “fair” if the patient
still needed non-narcotic analgesics, and 3) “poor” if residual pain
required narcotic use or interfered with normal activities. Using these
criteria, 50, 9, and 41% of the patients had good, fair, and poor pain
relief, respectively, from the procedure. The investigators noted that
certain types of SCI-pain syndromes responded better. For example, 74%
of patients with pain below the level of injury had good pain relief.
Complications were reported in 16 patients, including cerebrospinal
fluid leaks and loss of function and sensation. For example, one patient
who was able to walk with mechanical support before the procedure lost
the ability afterwards.
7) In 1988, Dr. Stephen Powers et
al (USA) summarized the results of treating 40 patients with various
types of central pain with laser-generated DREZ lesions. Of these
patients, 11 had paraplegia, nine from thoracic injuries and two from
cauda-equina injuries. Patients were followed for periods ranging from
four months to over five years. Five of the 11 individuals with
paraplegia had good pain relief from the procedure; the others did not.
Certain types of SCI pain appeared to be more responsive to DREZ
lesioning, and those with thoracic injuries generally had better
outcomes. Several individuals out of the 40 treated experienced motor
and sensory abnormalities as a result of the procedure.
8) In 1990, Dr. Blaine Nashold and
colleagues (USA) reported the results of treating 18 individuals with
paraplegia, who had delayed pain associated with the development of a
syringomyelia spinal cyst months to years after injury. Fourteen
patients had a single cyst, and four had two. After surgically exposing
the relevant area of the cord, restrictive adhesions were cut, the cyst
opened and drained, and radiofrequency DREZ lesioning carried out. With
follow-up averaging 3.5 years, pain relief was evaluated using the
following criteria: 1) Good: no analgesics needed and no
limitation of activities due to pain, 2) Fair: no narcotics
needed and no limitation of activities due to pain, and 3) Poor:
narcotics required and/or activities limited by pain. Using these
criteria, 14 and four patients experienced good and fair pain relief,
respectively.
9) In 1990, Dr. Ronald Young (USA)
summarized his experience using DREZ lesioning to alleviate pain in 78
patients over a seven-year period. The pain was caused by a variety of
disorders, including 20 with SCI and six with cauda-equina injuries. As
technology evolved, three different DREZ-lesioning methods were
employed. Initially, a radiofrequency method was used to treat 21
patients (group 1). Then a laser approach was employed to treat 20
individuals (group 2). Finally, a radiofrequency procedure using a
smaller electrode was adopted for 37 patients (group 3).
Dr. Young noted strengths and weaknesses
for the different procedures. For example, the initial radiofrequency
method (i.e., group 1) had difficulty penetrating spinal-cord associated
tissue due to the electrode’s larger tip size and produced lesions
lacking consistency in size. These problems appeared to be minimized
when the smaller electrode was later employed. The laser approach had a
number of benefits, including 1) not having to actually touch the spinal
cord, thereby avoiding physical trauma, and 2) creating closely spaced
lesions. However, deficiencies were also noted. For example, small
amounts of cerebrospinal fluid on the cord could significantly alter
lesion size.
Of the 78 patients treated, 62% had
satisfactory pain relief defined as at least a 50% reduction in pain,
stopping of narcotic analgesic use, and better functional ability. Using
these criteria, 55% on the individuals with SCI and 83% of those with
cauda-equina injuries had pain relief. Comparing the three DREZ methods,
67, 45, and 68% of the group 1 (radiofrequency), 2 (laser), and 3
(radiofrequency – small tip), respectively, obtained effective pain
relief. A variety of complications were observed, including a loss of
function and sensation. Specifically, in group 1, 52% of the patients
had complications; in group 2, 15%; and in group 3, 8%. This data
indicated that the small-tipped, radiofrequency device produced the best
results with the least complications.
10) In 1993, Dr. Robert Edgar and
colleagues (USA) reported their experience using computer-assisted DREZ
lesioning on 46 patients with central pain due to SCI. Noting that
significant numbers of individuals failed to achieve adequate pain
relief with traditional DREZ procedures, the investigators developed a
computer-assisted process in which electrophysiological assessments were
made in the DREZ at and above the injury area. After being computer
analyzed, the electrophysiological activity associated with each
specific location was categorized as normal or abnormal. Assuming that
the areas of abnormal activity were associated with pain generation,
DREZ-lesioning targeted these areas. In other words, areas of normal
activity were left alone, which would minimize unneeded, potentially
function-compromising neurological damage. Conversely, the area
subjected to DREZ lesioning was expanded if abnormal activity was
demonstrated to extend beyond the area that would have been normally
targeted. Patients were followed for an average of 44 (range 2- 96)
months. Using the computer-assisted process, 50-100% pain relief
occurred in 92% of patients, and 100% pain relief occurred in 84% of the
patients.
11) Reported in 1995, Dr. John Sampson
and associates (USA) summarized the outcomes of DREZ-lesioning over a
14-year period for 39 individuals with intractable pain due to trauma of
the conus medullaris or cauda equina. Thirty-one patients were males,
and age ranged from 17-66 years. Patients were followed for an average
of three years. Pain relief was classified as good if the
patients required no pain-killing analgesics, fair if pain was
significantly reduced but there was still a need for non-narcotic
medication for pain that no longer interfered with daily-living
activities, and poor if the previous criteria were not met. Using
such classification, 54 and 20% of the patients had good and fair pain
relief, respectively, from the procedure. About 20% of the patients had
serious complications, including weakness, bladder and sexual
dysfunction, cerebrospinal fluid weak, and wound infection.
12) In 1996, Dr. Stefan Rath and
colleagues (Germany) reported the results of treating 51 patients with
pain generated from variety of spinal and peripheral nerve lesions using
the DREZ procedure. Of these patients, 22 (18 males and 4 females) had
SCI. Their age ranged from 17-74 (average 47) years, and 20 had thoracic
and two lumbar injuries. Patients had been injured by fall (10), traffic
accidents (8), skiing (3), and gunshot (1). Seven patients also had
syringomyelia cysts at the level of injury, which were drained in
addition to the DREZ lesioning.
After the procedure, patients were
followed for periods of time ranging from 10 months to 13 years, rating
their postoperative pain levels as a percentage of preoperative levels.
A greater than 75% reduction in pain was defined as good, a 25-75%
reduction considered fair, and less than 25% reduction defined as poor.
Using these classifications, 12 (55%) of the patients with SCI had good
or fair continuing pain relief. Five of the seven individuals with
syringomyelia cysts reported poor outcomes. After the procedure, several
patients reported new paraesthesias
(i.e., tingling, burning, numbness sensation of the skin).
13) In a 1997 paper, Dr. Rath’s
team further summarized their experience using DREZ lesioning in now a
total of 68 patients, including 23 with SCI. Results were similar to
those reported previously, specifically, 12 patients noting continuous
good or fair pain relief.
14) Reported
in 1999, Dr. Milan Spaic et al (Yugoslavia) used DREZ lesioning
to treat six males with SCI with neuropathic pain. Ages ranging from
25-35 years, all had sustained thoracic or lumbar level injuries
(T10-L1) due to gunshot wounds. In a function-restoring effort, omental
transposition had been performed on all six 4-17 months after injury. As
discussed elsewhere, with this procedure, the omentum, a highly
vascularized, nutrient-rich, fatty tissue covering the gut, is
surgically tailored to create a tissue pedicle of sufficient length so
it can be sutured over the spinal-cord injury site. For these patients,
omental transposition did not improve function nor did it inhibit pain
development.
Because of this failure, 30-60 months
after omental transposition, the patients underwent DREZ-lesioning. In
this surgery, the omentum was removed, DREZ-lesioning carried out, and
the omental tissue reattached. Based on follow-up periods ranging from
7-12 months, four of the six patients had complete pain relief, and two
had 80% pain relief, sufficient enough to eliminate pain medications.
Some existing sensation was compromised by the DREZ surgery.
15) In 2001, Dr. Marc Sindou, a
pioneer in developing the DREZ procedure, and colleagues (France)
provided an analysis of the long-term results obtained by treating over
a 19-year period 44 patients with neuropathic pain resulting from spinal
cord or cauda equina injuries. Age averaging 46 years, 32 patients were
males and 12 females. Injury was cause by road accidents (16), falls
(11), industrial accidents (5), gunshot (4), skiing (2), and other (1).
The level of injury was cervical in three patients, thoracic in 22,
thoracic-lumbar in five, and lumbar in 14. Pain relief was considered
good if the patient estimated at least a 75% reduction in pain, fair if
a 25-75% reduction, and poor if a 25% or less reduction.
Three months after the DREZ surgery, 66,
20, and 14% of the patients reported good, fair, and poor pain relief,
respectively. Thirty patients were followed for longer periods ranging
from 1-20 (average 6) years. Of these, 60% reported good pain relief,
20% fair results, and 20% poor pain relief. The investigators noted that
those individuals with lower level injuries and those with incomplete
injuries appeared to get more enduring benefit. Few neurological side
effects were noted.
16) In 2002, Dr. Scott Falci and
associates (USA) reported their experience using an electrophysiological
guidance system to improve DREZ-lesioning outcomes for central pain in
41 patients with SCI. Basically, this system was used to identify areas
of electrical hyperactivity indicative of abnormal pain processing. Such
identification would allow better targeting of the DREZ lesions. Patient
age ranged from 19-72 (average 46) years, and 38 were men and three
women. All patients had sustained either thoracic or thoracic/lumbar
injuries and had started experiencing pain within a year of injury, most
soon after injury. The average time lapsing between initiation of pain
and surgery was 62 months.
The investigators concluded that the
guidance system greatly improved outcomes. Using the system, 84% of the
patients reported 100% pain relief. However, the majority experienced
some loss of sensation in areas affected by the spinal-cord area being
lesioned. In addition, the procedure resulted in new motor deficits in
five patients.
17) Between 1986 and 2006, Dr. Yucel
Kanpolat et al (Turkey) treated 55 patients with pain resulting from
a variety of neurological causes, including 17 with SCI. Of these
patients, 44 were men and 11 women with an average age of 46 (range
24-74) years. Two different DREZ surgical procedures were used. In 44
patients, the conventional DREZ-lesioning approach was employed, while
in 11 patients, the DREZ surgery targeted a nearby spinal area called
the nucleus caudalis. Patients were followed for periods ranging from
six months to 20 years. One year after surgery, 69% of the
conventionally treated patients and 62% of the alternatively treated
patients reported satisfactory pain relief. A patient in each group died
after surgery.
18) In 2011, Dr. F. Ruiz-Juretschke
and associates (Spain) summarized the outcomes of treating 18 patients
with DREZ-lesioning over a 15-year period. The most common disorder
within this group was brachial plexus avulsion; only two had SCI. Seven
patients were men and 11 women. Age ranged from 27-77 (average 52)
years. The duration of pain before surgery averaged six years. Patients
were followed for periods ranging from 6-108 (average 28) months.
Subjectively evaluated by the patient, pain relief after DREZ lesioning
was classified as excellent if pain was absent, good if
pain relief exceeded 75%, moderate if it was between 25-75%, and
poor if it was less than 25%. Using this classification, long-term pain
relief was deemed excellent in three patients, good in six, moderate in
three, and poor in six. After surgery, 67% of the patients could reduce
their pain medications, and 28% were able to go back to work. The best
results were observed in patients with brachial plexus avulsion. The
investigators reported neurological complications in four patients.

OTHER PAIN-MANAGEMENT TECHNIQUES
Acupuncture
As discussed
elsewhere, acupuncture has considerable therapeutic relevance for SCI,
including even restoring some function after injury. In addition, the
therapy can influence pain-processing neural pathways and
neurotransmitter systems. For example, it stimulates muscle sensory
nerves, which send messages to the spinal cord, midbrain, and pituitary,
which, in turn, releases pain-reducing molecules such as endorphins and
cortisol-producing hormones. It has been shown in rabbits that the
effects of acupuncture-induced analgesia can be transferred to other
rabbits through the transfer of cerebrospinal fluid.
Because acupuncture
has been extensively used in the general population to treat pain from a
variety of causes, several studies have been initiated to evaluate its
ability to reduce SCI-associated pain:
1) In 2001, Dr.
Sangeetha Nayak and associates (USA) reported the results of
treating 22 individuals with SCI for pain. Age averaged 43 years, and
68% of the participants were men. The time since injury and duration of
pain both averaged ~8.5 years. Cause of injury included motor vehicle
accidents (10), sporting accidents (5), gunshot or stabbing (4), falls
(2), and other (1). Eight, 13, and 1 had cervical, thoracic and
lumbar/sacral injuries respectively.
All subjects had 15
acupuncture sessions over a 7.5-week period. In each session, 6 to 14
acupuncture points were needled. Certain points were always needled,
including a key point located in the Governor acupuncture meridian
between the C-7 and T-1 vertebrae (see figure). Other points were
selected based on locations of pain reported by subjects, as well as
their response to previous sessions.
Subjects rated their
pain intensity using a 0-10 pain scale in which 0 corresponded to no
pain and 10 to pain as bad as it can get. To be eligible for the study,
all subjects had to have a 5+ pain level for at least six months before
enrollment. At various times before and after treatment, subjects were
asked to rate their present pain, average pain for the
past two weeks, and worst pain experienced during the preceding
two weeks.
Secondary assessments
focused on 1) pain-associated general health issues, such as
sleeping, appetite, range of motion, etc. 2) the degree to which pain
interfered with daily activities of life, 3) mood changes,
such as depression or anxiety, 4) perceived psychological well being,
and 5) treatment expectations.
Using the 0-10 pain
scale, average pain decreased from 6.9 before to 5.4 after treatment, a
reduction which persisted for some time. More specifically, 18% of the
subjects reported a significant reduction in pain (defined as at least a
3-point decrease in pain levels), 27% reported a moderate reduction
(2-3-point decline), 36% reported minimal pain relief (< 2-point
decline), and 18% reported an increase in pain.
Those who responded
better to acupuncture tended to have pain located above the injury, an
incomplete injury, or musculoskeletal, as opposed to, central pain.
Subject expectation of pain relief that would accrue did not correlate
with the pain relief they actually got; i.e., believing in acupuncture’s
potential did not result in more benefit.
Regarding secondary
assessments, there was a reduction in various pain-associated symptoms
(e.g., sleep difficulties) and less interference in activities of daily
living. No improvements in anxiety or depression were noted. Improvement
in psychological well being, especially in perceived vitality/energy
levels, was documented.
2) As reported in
2003, Dr. Linda Rapson and colleagues (Canada) developed an
electr o-acupuncture
protocol to treat SCI-associated neuropathic pain. Under this protocol,
acupuncture needles were inserted in three points of the Governor
Meridian (points 18, 20, 21; see illustration) in the scalp o-acupuncture
protocol to treat SCI-associated neuropathic pain. Under this protocol,
acupuncture needles were inserted in three points of the Governor
Meridian (points 18, 20, 21; see illustration) in the scalp midline and in a fourth point called the Yin Tang located between the
eyebrows. After insertion, the needles were electrically stimulated for
30 minutes. Patients were initially treated five times per week, and
treatment was continued until full relief of pain was accomplished or no
further benefits accrued.
midline and in a fourth point called the Yin Tang located between the
eyebrows. After insertion, the needles were electrically stimulated for
30 minutes. Patients were initially treated five times per week, and
treatment was continued until full relief of pain was accomplished or no
further benefits accrued.
 The
investigators retrospectively reviewed the medical charts of 36 patients
with spinal cord dysfunction (22 with traumatic injuries) who were
treated for pain using this electro-acupuncture protocol over a
five-year period. Twenty-three and 13 were men and women, respectively,
and age ranged from 17 to 75 years. Of the 36 patients identified, 24
benefited from treatment, including 18 who experienced pain relief after
only one treatment. No adverse side effects were observed The
investigators retrospectively reviewed the medical charts of 36 patients
with spinal cord dysfunction (22 with traumatic injuries) who were
treated for pain using this electro-acupuncture protocol over a
five-year period. Twenty-three and 13 were men and women, respectively,
and age ranged from 17 to 75 years. Of the 36 patients identified, 24
benefited from treatment, including 18 who experienced pain relief after
only one treatment. No adverse side effects were observed
3) In 2001, Dr.
Trevor Dyson-Hudson and colleagues (USA) evaluated the use of either
acupuncture or Trager bodywork (below) to treat shoulder pain in
individuals with SCI who used a manual wheelchair. This investigation
was initiated by studies suggesting that as many as 68% of those with
SCI have such pain due to pushing a wheelchair, transfers, and various
activities of daily living. Fourteen men and four women were recruited
with age averaging 45 (range 28-69) years. On average, they had been
injured ~15 years and had shoulder pain for ~6 years. Cause of injury
was motor vehicle crashes (9), falls (3), gunshot (2), diving accidents
(1), and surgical/medical complications (4). Subjects reported doing
about 10 wheelchair transfers per day.
Subjects were
randomized to receive either 10 acupuncture or Trager treatment sessions
over a five-week period. In each acupuncture session, various points
associated with upper extremity pain and areas of tenderness were
needled. In the Trager sessions, gentle motions were used to loosen
joints, ease movement, and release chronic pain patterns. The subjects
were also taught Mentastic exercises (from “mental gymnastics”) to help
recognize movements or tension patterns that may lead to pain.
Various assessments
of pain were recorded periodically before, during, and after treatment,
including the “The Wheelchair User’s Shoulder Pain Index” (WUSPI). With
this index, subjects were asked to report shoulder pain intensity for 15
activities of daily living (e.g., transfers, etc) using a 0 (no pain) to
10 pain (worst possible pain) scale for each activity. The scores for
all 15 activities were combined into a single score (150 being the
highest possible score).
By the end of the
treatment period, WUSPI pain levels had decreased ~54% for both the
acupuncture- and Trager-treated individuals. Although pain started
trending upwards after treatment was discontinued for the acupuncture
subjects, it continued to decline somewhat for Trager subjects.
 The
investigators speculate that this continued decline was due to the
Trager emphasis on movement reeducation, which would have a lasting
influence even after treatment was stopped. Overall, 89% and 100% of the
acupuncture- and Trager-treated individuals, respectively, reported less
shoulder pain after treatment. The
investigators speculate that this continued decline was due to the
Trager emphasis on movement reeducation, which would have a lasting
influence even after treatment was stopped. Overall, 89% and 100% of the
acupuncture- and Trager-treated individuals, respectively, reported less
shoulder pain after treatment.
4) Because the
previous study did not have a placebo-control group, the investigators
initiated a somewhat similar investigation in 17 subjects with shoulder
pain who were randomized to receive either active or sham
acupuncture, which needled supposedly inactive, nearby skin areas. In
this more rigorously designed, double-blind study, neither the subject
nor the evaluator knew who received active versus sham treatment. Of the
17 subjects, two were females and 15 males; 6 and 11 had tetraplegia and
paraplegia, respectively; age averaged ~39 years; and the time lapsing
since injury averaged ~11 years.
Subjects received 10
treatments over a five-week period. For the acupuncture-treated
subjects, six points in the vicinity of the shoulder pain and two points
further away were needled. Also needled were points of tenderness that
did not correspond to classical acupuncture points. The needles were
manually stimulated several times (e.g., twirling) and left in for 20
minutes. In the case of the sham subjects, needles were inserted in
supposedly inactive areas somewhat near the true acupuncture points.
Also, the needles in the sham-control subjects were more shallowly
inserted and not manipulated.
Once again, the
primary measure of pain was “The Wheelchair User’s Shoulder Pain Index,”
which was assessed before, right after, and five weeks after treatment.
Using this index, shoulder pain in acupuncture-treated subjects
decreased 66% compared to 43% for the sham-treated individuals.
In the case of the
more general 0 (no pain) to 10 pain (worst pain possible) scale,
shoulder pain in acupuncture-treated individuals decreased from 5.0 to
2.5 after treatment, and in the sham subjects declined from 4.3 to 3.6
after treatment. Seventy-five percent of the individuals in the
acupuncture group reported a clinically meaningful reduction in pain
(defined as a 30% reduction) five weeks after treatment compared with
only 25% for the sham-treated individuals.
Although active
acupuncture resulted in a greater reduction in pain compared to sham
treatment, due to the relatively small sample sizes, the differences
between the two groups were not statistically significant. As discussed
by the investigators, the use of sham acupuncture points in clinical
trials has been problematic because they are
not neutral controls. Although not as effective as true acupuncture
points, sham points also evoke physiological responses through different
mechanisms and, therefore, are questionable as a control comparison.
5) In
2011, Drs. Cecilia Norrbrink and Thomas Lundeberg (Sweden)
reported the results of treating 30 individuals with SCI and neuropathic
pain for six weeks with either twice-weekly acupuncture or massage
therapy (15 in each group). The acupuncture group was composed of 12 men
and 3 women with an average age of 47. The time since injury averaged
~12 years, and five had tetraplegia. The massage group had relatively
similar composition.
In each
session 13-15 acupuncture points were needled, including several points
that were stimulated by electro-acupuncture. The investigators used a
variety of pain assessments involving a 0 (no pain) to100 (worst
possible pain) scale. A clinically meaningful reduction in pain was
defined as a decrease of 18 units on this 0-100 pain scale. Using this
scale, subjects periodically rated their present, general,
and worst pain intensity, as well as pain unpleasantness
and other measures.
Ratings
for general pain, present pain, and pain unpleasantness all had a
statistically significant decline at the end of acupuncture treatment.
Specifically, general pain decreased from 63 to 48 after treatment,
present pain decreased from 59 to 40, and pain unpleasantness decreased
from 70 to 47. Although declines were also observed for the massage
group, they were not as large and did not reach statistical
significance.

Hypnosis
“The trick is not
minding that it hurts” – Peter O’Toole in Lawrence of Arabia
Hypnosis is a
trance-like state of consciousness distinguished by increased
susceptibility to suggestion, relaxation, and imagination. Based on
evidence that it can significantly alter the perception of pain, Dr.
Mark Jensen and colleagues (USA) have examined its potential to
reduce the unique pain associated with SCI (1-6). If effective, hypnosis
would avoid the many adverse side effects associated with pharmaceutical
or surgical approaches.

As in all life, our
perception molds our reality. For example, a long wait in an
express-checkout lane can be viewed as either a pain-in-the-behind
hassle or an opportunity to read a tabloid magazine on display.
Likewise, if you get slammed playing wheelchair rugby, you’ll probably
forget your headache for awhile. The attention you direct to the pain is
energy that fuels it. Basically, the goal of hypnosis is to cut off
this fuel-line of consciousness by placing the pain within a different
context, deemphasizing your focus on it, or directing your attention
elsewhere.
1) In 2000, Dr.
Jensen and colleague Dr. Joseph Barber reported the results of treating
four individuals with SCI with four sessions of hypnosis. Before
treatment, participants were evaluated for 1) their responsiveness to
hypnosis, 2) pain intensity using a 0-10 pain scale with 0 corresponding
to no pain and 10 corresponding to the most intense pain imaginable, and
3) the degree to which pain interfered with their sleep using another
0-10 scale in which 0 corresponded to no disturbance and 10 to unable to
sleep. Participants also kept daily diaries assessing pain and sleep
interference for the previous day. They were encouraged to practice self
hypnosis daily between treatments and afterwards using a 20-minute
audiotape prepared during the first session.
After hypnotic
induction, participants were given various suggestions for pain relief,
examples of which are shown below. Future sessions were tailored based
on patient responsiveness to the suggestions in the initial session. At
the end of each session, patients were told that they would be able to
recreate their hypnotic state by taking and holding a deep breath and
listening to the audiotape specifically tailored for them.
EXAMPLES OF
HYPNOTIC SUGGESTIONS
|
1) Direct suggestions for
decreased pain |
Example: “You notice that as you
relax, as you feel more comfortable, you feel less and less
pain, almost as if the pain were going away, or getting
smaller.” |
|
2) Direct suggestions for
increased comfort |
Example: “You can feel more and
more comfortable…” |
|
3) Replacement of pain with
other sensations |
Example: “You can notice how any
feelings of pain or discomfort can change…to other feelings…
feelings that are not unpleasant…that are more comfortable…like
warmth or a very pleasant tingling sensation…” |
|
4) Ability to ignore pain |
Example: “As your pain continues
to decrease, as you build this barrier between pain and your
experience, it is almost like it is muffled…you notice it less
and less.” |
|
5) Displacement |
Example: “The pain and
discomfort that you usually experience can now be directed and
moved to a different part of the body.” |
|
6) Hypnoanaesthesia |
Example: “It is now time to
anaesthetize the site of your pain. Notice how naturally, how
easily the area of pain and discomfort is being engulfed in a
psychological anaesthesia.” |
Patient experiences
are summarized below:
Patient 1,
a 65-year-old woman who sustained an incomplete C-5 cervical injury 43
years earlier, had foot pain that would build up during the day to a
level of 6.5 on the aforementioned 0-10 pain scale. This interfered with
her ability to sleep. She was rated a 3 out of a possible 5 in terms of
hypnotic susceptibility. Her pain levels dropped to 3.8 during the five
days after her final session. Likewise, her sleep-interference rating
dropped from 6.2 to 3.4 during the same period. Because she did not
continue to practice self hypnosis, her pain intensity and sleep
disturbance returned to pretreatment levels when assessed at two months
and one year after treatment.
Patient 2,
a 28-year-old male who sustained a complete C-5 injury 10 years earlier
due to a motor vehicle accident, had stinging pain in his legs and
hands. His hypnotic responsiveness was rated 5 out of 5. His pain
intensity decreased on average from 2.0 before treatment to 1.5
afterwards, and sleep interference decreased from 3.0 to 1.0. After
treatment, he practiced self hypnosis daily using the prepared
audiotape. Decreases in pain levels and sleep interference were
maintained at two months and one year after the initial treatment.
Patient 3,
a 37-year-old male who sustained a C-4/5 level injury due to gunshot 14
years earlier, had lower back pain rated as 5 in intensity on the 0-10
pain scale. His hypnotic susceptibility was 5 out of 5. Treatment and
daily self hypnosis reduced his pain to 0.5 and eliminated sleep
interference. However, pain intensity and sleep interference started to
increase four months after treatment because he stopped his daily
practice after losing his audiotape.
Patient 4
was a 42-year-old woman who had been injured at the T12/L1 level 17
years earlier due to a fall. She experienced constant and uncomfortable
electrical sensations in her legs, which she rated as a 4.5 on the 0-10
pain scale. Her hypnotic susceptibility was 4 out of 5. With treatment
and self-hypnosis practice, her pain intensity dropped to 2.0, and her
sleep interference decreased from 3.5 to 1.5. Although before treatment,
she was always aware of her pain, afterwards there were periods in which
she was completely unaware of it.
From this preliminary
data, the investigators concluded that hypnosis has the potential to
reduce pain and improve sleep quality in varying degrees for some
individuals with SCI. All patients reported decreases in pain and sleep
disturbance after treatment. Those who practiced self hypnosis
maintained or even improved on these treatment gains.
2) In 2005, Dr.
Jensen and colleagues reported the results of using hypnosis to treat
pain in 33 individuals with SCI (13), multiple sclerosis (10),
amputation (7), and other disabilities (3). Participant age averaged
51(range 28-79) years, and 18 were women and 15 were men. The treatment
consisted of 10 hypnosis sessions spaced at time intervals ranging from
daily to weekly sessions depending upon individual availability.
In each session, one
of five pain-relieving suggestions was given after hypnotic induction,
involving 1) decreased pain, 2) deep relaxation, 3) hypnotic analgesia,
4) decreased unpleasantness, or 5) sensory substitution. After the
initial suggestion, participants would be returned to a fully alert
state and then after another hypnotic induction, be provided the next
suggestion. The process was repeated until all five suggestions had been
administered. Unlike the previous study in which later sessions were
tailored based on the responsiveness to the suggestions in the initial
sessions, all sessions incorporated every suggestion. Although tailoring
appears more effective, this procedure was adopted to standardize the
intervention for the sake of better comparing study results. At the end
of each session, participants were given posthypnotic suggestions to
facilitate self-hypnosis practice and to promote extended pain-relief
benefits.
The study’s primary
outcome measure was average pain intensity, using the aforementioned
0-10 pain scale. This was assessed before treatment, after the 10
sessions were completed, and three months later. The 27 individuals who
completed all ten sessions reported an average 21% reduction in pain. Of
these 27 participants, 10 reported a clinically meaningful, 30% or
greater reduction in pain. Much of the pain reduction still existed
three months after treatment. Although it was difficult to make
meaningful conclusions given limited sample sizes for each disability,
participants with amputation seemed to have the greatest pain relief.
Specifically, they reported a 43% average reduction in pain compared to
17% for SCI and 10% for multiple sclerosis.
3) Building upon this
study, in 2008, Dr. Jensen’s investigative team summarized the
long-term, pain-relief benefits accruing to 26 individuals with
pain, now including 12 with SCI, 8 with MS, 5 with amputation, and 1
with post-polio syndrome. Average age was 50 (range 28-79) years, and 14
of the 26 participants were men. As described above, each individual had
been treated with 10 hypnosis sessions. To maintain pain-amelioration
benefits over time, participants were encouraged to regularly practice
self-hypnosis by using a post-hypnotic cue given at treatment and, in
some cases, a practice tape provided at the 3- or 6-month follow-up
assessment.
Using the 0-10 scale,
pain levels were assessed 3, 6, 9, and 12 months after treatment. At
all follow-up periods, average pain intensity was lower than that
observed before initial treatment. The percentage of participants
reporting a clinically significant (i.e., >30%) reduction in average
pain intensity at these follow-up times were 27%, 19%, 19%, and 23%,
respectively. Although these reductions seem modest, the majority of
participants reported that they frequently used self-hypnosis, on
average 16-17 days per month. Apparently, even though pain intensity for
the whole day may have improved only slightly, substantial short-term
pain relief lasting several hours occurred. These results indicated that
self-hypnosis might be especially useful for pain flare-ups.
4) In 2009, Dr.
Jensen and associates reported the results of treating a 27-year-old
male Army Sergeant who had sustained a cervical C6, ASIA-B (see
appendix) incomplete injury from a gunshot to his neck. Because of
severe pain in his arms, he could not tolerate range-of-motion and
physical-therapy exercises. During such exercises, he rated this pain
as 10 (worst pain imaginable) on the 0-10 pain scale.
Because the patient
experienced adverse reactions to pain medications, hypnosis was tried.
Over a five-week period, he had 10 hypnosis sessions lasting 45-75
minutes each. At the beginning of each session, he was asked about his
current pain location, average pain intensity over the past day, and
current pain intensity. During the first two sessions, the patient was
given five specific pain-reduction suggestions, involving direct pain
reduction, relaxation, imagined anesthesia, decreased pain
unpleasantness, and replacement of pain with other sensations. Future
sessions were tailored based on his responsiveness to these suggestions.
In addition, suggestions were given concerning his overall healing,
progression in therapies, and increased self-confidence about his
eventual discharge from the hospital and return to civilian life. Each
session ended with post-hypnotic suggestions for continued self-hypnosis
practice. To facilitate his practice, audio recordings were prepared for
his use.
Due to hypnosis
treatment, the patient’s pain levels greatly decreased. As a result, he
could straighten his fingers (which was previously not possible due to
intense pain), and his hands lost their “claw-like” appearance.
Furthermore, he could now participate more fully in therapies with less
pain and was able to substantially reduce pain medications. At the end
of each session, his pain levels were never greater than 2 on the 0-10
scale. At a six-month telephone follow-up, the patient reported that his
sensitivity to pain had decreased considerably. At its worst, it was a
5-6 and at best, a 1.5-2 on this scale. He had continued to practice the
self-hypnosis skills learned during treatment.
5) In 2009, Dr.
Jensen et al summarized their treatment of 37 individuals with SCI
possessing chronic pain. These individuals were randomized to be treated
with either hypnosis or a biofeedback-relaxation technique. The study
was designed to distinguish hypnosis’ true pain-relieving ability from
any placebo effect. Unlike studies evaluating drug efficacy in which an
inactive agent can be readily used for comparison, it is difficult to
create a control for treatment modalities such as hypnosis in which
subjects will most likely know if they are being treated and, in turn,
report benefits skewed by that knowledge.
Because of this
difficulty, the investigators chose to select biofeedback relaxation as
a control treatment because, in part, it could be administered in a
fashion somewhat similar to hypnosis. In hindsight, they concluded that
it was a poor choice because it was not inactive but rather a modality
that brought some pain relief through mechanisms overlapping with those
of hypnosis (e.g., suggestive techniques). In other words, both the
hypnosis and control treatments brought about some pain relief, making
definitive conclusions on effectiveness more difficult.
Participant age
averaged 49.5 (range 19-70) years, and, 28 were men. Of the 37 initially
recruited and randomized to the two treatment groups, 28 completed the
10-treatment regimen. Similar to the procedures described earlier, in
the initial hypnosis sessions, participants were given five specific
pain-reduction suggestions, involving decreased pain, deep relaxation,
hypnotic anesthesia, decreased unpleasantness, and sensory substitution.
In turn, future sessions were tailored based on individual
responsiveness to these specific suggestions. Participants were
encouraged to practice self hypnosis between sessions, as well as after
completion of the 10 sessions by using post-hypnotic suggestions and
through listening to audiotapes recorded at the sessions. To make the
hypnosis and control study arms more comparable, a biofeedback
audiotape, which included a relaxation exercise, was also provided to
the control subjects.
Average daily
pain intensity was evaluated before and after treatment and three months
later. In addition, current pain intensity was measured before
and after each treatment session. Although the reduction in pain
intensity before and after each session was comparable for both
hypnosis- and biofeedback-treated individuals, hypnosis subjects
experienced a greater reduction in average daily pain intensity.
Specifically, their average daily pain decreased from 6.10 on the 0-10
pain scale before treatment to 5.05 afterwards to 4.93 three months
later. For the biofeedback group, average daily pain decreased from 3.38
before treatment to 3.17 afterwards but increased to 3.78 three months
later. Three months after treatment, 31% of the hypnosis subjects and
22% of the biofeedback subjects reported a clinically meaningful 30% or
greater decrease in their average daily pain relative to pretreatment
levels.
Interestingly,
participants experiencing neuropathic pain had greater pain reduction
from hypnosis than those with nonneuropathic pain (e.g., visceral,
mechanical spine, or overuse). 
Transcutaneous Electrical Nerve Stimulation (TENS)
Transcutaneous
electrical nerve stimulation (TENS) has been extensively used to treat
pain generated from a variety of causes, including neuropathic origins.
Basically, TENS devices transmit low-voltage, electrical impulses at
various frequencies through electrodes attached to the skin in areas
associated with pain. Although numerous studies document the
pain-relieving benefits accruing by using TENS devices, because few
studies were controlled, experts continue to debate the true efficacy of
the procedure.
Evidence suggests
that TENS may lessen pain through a variety of physiological mechanisms,
including 1) inhibiting the transmission of pain signals from the
peripheral nervous system into the spinal cord, and 2) increasing the
levels or influence of pain-reducing, neurotransmitters, such as
opioid-like endorphins.
Over the years,
several studies have focused on the potential of TENS to treat
SCI-associated pain, including the following:
1) In 1975, Drs.
Ross Davis and Richard Lentini (USA) briefly summarized the
preliminary results of using TENS to treat 31 subjects with various
forms of SCI-associated pain. Subject age varied from 23 to 68 years,
and injury levels ranged from the cervical C-3/4 to lumbar L-5 level.
Some pain relief was reported by 13 of the 31 subjects, those with
central pain accruing less benefit.
2) In 1978, Dr.
H.J. Hachen (Switzerland) reported the results of treating 39
individuals with SCI suffering from chronic intractable pain.
Twenty-five were men, and 14 were women; 32 and 7 had paraplegia and
tetraplegia, respectively; and 18 and 21 had sustained complete and
incomplete injuries, respectively. The duration of pain had ranged from
6 to 35 months. In the first week of treatment, TENS stimulation was
applied for six consecutive hours, and thereafter, the treatment
schedule was tailored to individual requirements. After a week of
treatment, 49% of the subjects reported almost complete and 41% slight
pain relief. After three months, these figures were 28% and 49%,
respectively.
3) In 2009, Dr.
Cecilia Norrbrink (Sweden) reported the results of treating 24
subjects with SCI and neuropathic pain with either high- or
low-frequency TENS. Subjects included 20 men and four women with an
average age of 47 (range 29-68) years. The time lapsing since injury
varied from 0.5 to 28 (average ~7) years. Thirteen, eight and three had
cervical, thoracic, and lumbar injuries, respectively.
Subjects were
assigned to be treated with either high- or low-frequency TENS for two
weeks. After a two-week washout period in which no treatment was
administered, treatment was reversed; i.e., the subjects who had
received high-frequency TENS now were given low-frequency treatment for
two weeks and vice versa. After being shown how to use the TENS device,
subjects were instructed to employ it at home three times a day, 30-40
minutes per session for two-weeks. The device’s four electrodes were
place adjacent to the spinal column in the area of injury.
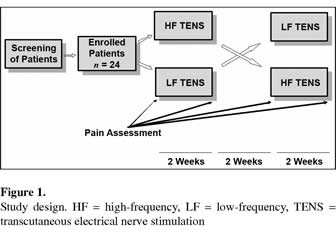
Various scales,
questionnaires, and other measurement were used to assess pain and
various factors affected by pain, such as mood, sleep quality, and life
satisfaction. For most assessments, no statistically significant benefit
accrued from TENS treatment, and no difference could be discerned
between high- and low-frequency approaches. However, using the global
pain-relief scale, in which subjects rated treatment as having no
effect, insufficient effect, rather good effect, good effect, or very
good effect, 29% and 38% of the subjects reported some benefit using
high-frequency and low-frequency stimulation, respectively. In addition,
in follow-up interviews, subjects reported increased relaxation,
decreased use of pain killers, increased ability to work, improved
mobility in shoulder joints, and improved sleep. After the study
concluded, 25% of the subjects requested a prescription for a TENS
device so they could continue treatment.

Healing
Touch
As discussed elsewhere, healing touch is “an energy
therapy in which practitioners consciously use their hands in a
heart-centered and intentional way to support and facilitate physical,
emotional, mental, and spiritual health.” It’s often used with other
therapies to accelerate healing.
Funded by the US Veterans Administration, Dr.
Diane Wardell and colleagues (USA) carried out a pilot study
evaluating the use of Healing Touch to treat SCI-associated neuropathic
pain.  Seven
male veterans with SCI, at least six-months post injury, were treated
with healing touch and compared with five who received a
non-healing-touch intervention. All subjects had more than one month of
neuropathic pain at a level greater than 5 on a scale ranging from 0 (no
pain) to 10 (worst possible). Seven
male veterans with SCI, at least six-months post injury, were treated
with healing touch and compared with five who received a
non-healing-touch intervention. All subjects had more than one month of
neuropathic pain at a level greater than 5 on a scale ranging from 0 (no
pain) to 10 (worst possible).
Certified haling touch practitioners administered
once-a-week sessions to each subject for six weeks. To avoid
practitioner variability, each subject was treated by the same healer
throughout the study. Based on the practitioner’s assessment of the
subject’s energy fields, the sessions were individualized; i.e.,
different techniques were used on different individuals. At the end of
the study, the primary caregiver (e.g., wife) for each subject was given
the option to be trained in healing touch so treatment could be
continued.
In addition to a variety of before-and-after
quantitative measurements of pain and other factors, qualitative
opinions were solicited from the subjects. Although the study was
inherently limited due to the small number of subjects and sessions, the
results suggested that healing touch “may be beneficial in the areas of
coping, pain management, decreasing fatigue, decreasing confusion,
increasing life satisfaction, and decreasing depression.” The
investigators believed the results warranted further, more definitive
studies.
Qualitative reactions from participants included:
Vitamin-D Supplementation
The importance of
vitamin-D to individuals with SCI has been discussed elsewhere. In
addition, vitamin-D-deficient individuals tend to have more chronic
pain, which may be alleviated by vitamin-D supplementation (1-10).
Although understandings are still evolving, evidence indicates that
vitamin D regulates the synthesis of key immune-system molecules (called
cytokines) implicated in pain-associated inflammatory responses.
It is uncertain how
much supplementation can lessen the unique pain experienced by
individuals with SCI. For example, it may help in overuse-related
shoulder pain but have little impact on neuropathic pain. We do not
know. Nevertheless, vitamin-D is a
nothing-to-lose-potentially-much-to-gain approach that, at minimum, will
enhance overall health.
Vitamin-D levels can
be measured through a simple blood test, which measures blood levels of
a specific vitamin-D derivative called 25-hydroxvitamin D. Levels above
30 nanograms (one billionth of a gram) per milliliter are considered
sufficient, between 20-29 ng/ml insufficient, and < 20 ng/ml inadequate.
Using these criteria,
it’s estimated that one billion people worldwide lack health-optimizing
vitamin-D levels. At special risk are the elderly and individuals with
dark skin pigmentation, especially those who live in cloudy, northern
latitudes. Wintertime sunlight possesses little of the wavelengths
needed to produce vitamin D in much of the northern U.S.
Scientists speculate
that people with physical disability are more likely to have
compromised vitamin-D levels because the disability limits time
outside in the sun. In the case of SCI, one study indicated that
individuals with chronic SCI were twice as likely to have deficient
vitamin-D levels compared to able-bodied individuals. Levels apparently
go down quickly after injury as demonstrated in another study showing
deficient vitamin-D levels in 93% of patients admitted to acute,
inpatient rehabilitation, including 21% who were considered severely
deficient with levels <10 ng/ml.
Numerous studies
suggest that vitamin-D deficiency has a key role in pain manifestation,
including the following:
1) Dr. Vasant
Hirani (UK) looked at the relationship of pain and vitamin-D levels
in 2,000+ adults aged 65 and over living in England, a northern, cloudy
country. Overall, as we age we become less efficient in synthesizing
vitamin D and converting it to its physiologically active form. Hirani’s
study indicated that moderate to extreme pain was present in 53% of
these elderly individuals and was correlated with poor vitamin-D status.
2) Dr. Wei Huang
and collaborators (USA) evaluated the effects of vitamin-D
supplementation in 28 veterans with chronic pain and low vitamin-D
levels. Age averaged 46 years, 18 were men, and 20 were African
Americans, reflecting this group’s tendency to possess suboptimal
vitamin-D levels. The average vitamin-D level was a deficient 18.6 ng/ml.
After receiving vitamin-D supplementation for three months, the average
level increased to 26 ng/ml.
Subjects rated their
pain before and after supplementation using a 0 to 10 pain scale, in
which 0 corresponded to no pain and 10 as worst possible pain. In
addition, a quality-of-life questionnaire was administered which
asked about physical functioning, the extent health interferes with
work, bodily pain, general health, vitality, social functioning, the
degree emotional problems interfere with work, and mental health.
Finally, another questionnaire was used to assess sleep quality, which
is frequently compromised by pain.
Using the 0-10 scale,
average pain levels decreased from 7.1 to 5.7 after supplementation.
Overall, subjects reported fewer areas of pain and a decreased use of
pain medications. In addition, the results indicated an improvement in
most of the quality-of-life components listed above, including
the pain component. Finally, sleep improved after supplementation, e.g.,
subjects took less time to get to sleep and slept longer. The
investigators concluded that “vitamin-D supplementation in veterans with
multiple areas of chronic pain can be effective in alleviating their
pain and improving sleep, and various aspects of quality of life.”
3) Individuals with
SCI often experience neuropathic pain due to neural-tissue damage.
Although studies focused on using vitamin-D to lessen neuropathic pain
are limited, Drs. Paul Lee and Roger Chen (Australia) examined
vitamin-D’s potential to relieve pain in 51 vitamin-D-deficient patients
with diabetes. Common in individuals with SCI, diabetes frequently
causes pain-generating, peripheral nerve damage. Three months of
supplementation increased vitamin-D levels in subjects from an average
of 18 to an average of 30 ng/ml. Several measures of pain were assessed
before and after supplementation, including a 0 (no pain) to 5
(excruciating pain) scale. Using this scale, pain levels decreased from
3.3 before supplementation to 1.7 afterwards, a 48% reduction.

Emotional Freedom Technique
The Emotional Freedom Technique (EFT) is
a form of acupressure-assisted exposure therapy, where you tap on
various acupressure points while focusing on pain-associated
emotional issues. Basically, the technique catalyzes the release of
negatively charged, stuck emotions.
EFT has successfully treated issues that have
yielded reluctantly, at best, to years of psychotherapy or
medication. Furthermore, because virtually all chronic disorders
have mind-body correlates, EFT has the potential to lessen physical
symptoms and pain, and pave the path to healing. EFT is a
self-healing and -empowerment technique that people can learn to do
by and for themselves. It has no negative side effects.
EFT is based on two key energy-medicine tenets.
First, we have an acupunctural system of points and meridians that
regulate the flow of life-force energy throughout our bodies. As an
analogy, view the meridians as a pipeline through which the energy
flows, the acupuncture points as periodically placed,
flow-controlling valves, and the acupuncture needles as the socket
wrench that opens the valves. [With EFT, instead of needles, the
pressure of tapping fingers regulates the flow.] Overall, each of us
has a unique energy flow that is optimal for our health, and when
this flow gets off-kilter for any mind-body-spirit reason, we become
compromised. Acupunctural theory believes that pain is often a
symptom of stuck energy.
Secondly, emotions are a function of our
internal energy flows. When our energy flows are weak or blocked, we
may feel tired, cranky, irritable or easily triggered. When our
energy flows are strong and open, we feel more fresh, present,
alive, loving, and joyful. By crimping energy flow and distribution,
negative emotions become psychosomatic baggage. Although we may not
appreciate the influence of these, often pushed-down, emotions in
everyday life, they are there, gnawing away at our ability to
function optimally. EFT releases the energy behind these emotions.
The heavy emotional suitcase we have been dragging through the long
concourse of life becomes a light carry-on of non-charged memories.
Overall, EFT-generated benefits include 1)
desensitizing negative emotions and associated physical reactions;
2) releasing mood-altering neurotransmitters and hormones; 3)
triggering the relaxation response; 4) interrupting stuck or
limiting behavioral patterns/mind-sets; 5) resetting the body’s
internal electrical system; 6) initiating energetic, perceptual,
cognitive, and physical shifts; and 7) inducing feelings of joy,
satisfaction, relaxation, peacefulness, and well-being.
EFT can blunt the impact of many of life’s
“slings and arrows of outrageous fortune” that chip away at our
spirit, ranging from the minor to the all-consuming. So to speak,
EFT is the lint brush that wipes away the unneeded clumps of
emotional lint that cling to us and cumulatively drag us down over
time.
The suffering, anguish, or anxiety associated
with major issues, like addiction, intense phobias, childhood abuse,
trauma, or post-traumatic stress, often let up in response to EFT.
On occasion, deep-seated, life-compromising issues have been quickly
resolved with EFT. The emotional balloon swollen with negative
charge is punctured and deflated.
Procedures: Given their often profound
results, the basic EFT procedures are amazingly simple and can readily
be picked up after a few short demonstrations. The use of EFT to
specifically relieve pain is summarized in the book Freedom at your
Fingertips compiled by Ron Ball (2006). Another good starting point
is the website
www.eftuniverse.com, which lists many resources, training
opportunities, and practitioners.
As summarized in the Table, you tap on key
acupuncture points while focusing on a specific issue. These points are
specifically selected because they are located at the end of various
acupuncture meridians. If you have limited finger mobility, use your
hands or just visualize the tapping.
|
BASIC EFT PROCEDURES
Preliminary
1) Start by picking the
issue, attempting to be as specific as possible. For general,
amorphous issues, dissect them into component parts and work on
each separately.
2) Assess issue intensity on
a scale from zero to 10 (most intense).
3) Create a reminder phase
to repeat while tapping. For example, if you have nagging
shoulder pain, your reminder phrase might be just “shoulder
pain.”
4) Locate the EFT “tender
spot” by going to the base of the neck where a tie is knotted,
and then go down three inches and over three inches. This area
is sometimes tender when rubbed because of lymphatic
congestion.
5) While rubbing the tender
spot, state the following, for example, affirmation three times
“Even though I have this throbbing pain in my right shoulder, I
deeply and completely accept myself.”
Tapping Sequence
Using several fingertips,
tap 7-10 times at each of the indicated locations (see
illustration) while repeating your reminder phase. The tapping
points proceed down the body, making them easier to memorize.
Face and Body:
1) Beginning of eyebrow on each side of nose, 2) Side of eyes,
3) Under each eye, 4) Under the nose, 5) Middle of chin, 6)
Beginning of collarbone where the sternum and first rib meets,
7) Four inches under each arm, and 8) One inch below each
nipple.
Hands & Fingers:
Tap the 1) outside cuticle edge of your thumb at the base of the
thumbnail, 2) thumb-facing edge of each finger (except ring
finger) at fingernail base, and 3) the fleshy outside edge of
the palm used to deliver a karate chop (To save time, tapping
can be consolidated, e.g., the outside edge of your right thumb
can be used to tap on the outside edge of your left thumb, etc.)
A longer EFT version
includes tapping on the hand’s gamut point (see
illustration) while carrying out various eye movements,
counting, and humming tunes. Although sounding strange,
different parts of the brain are stimulated with each of these
actions.
Finally, reassess the
intensity of the issue again and repeat the cycle. |

Exercise
Studies suggest that
exercise can reduce not only SCI-associated shoulder pain but perhaps
neuropathic pain:
1) In 1999, Dr.
K.A. Curtis’s investigative team (USA) examined the impact of a
six-month exercise program on shoulder pain in 42 wheelchair users,
including 35 individuals with SCI. Of the 42, 35 were men, age averaged
35 years, and the average duration of wheelchair use was 14 years. All
subjects with SCI had injuries at the cervical C6 level or lower.
Subjects were equally randomized into treatment and control groups. The
treatment group received instruction in five shoulder exercises, which
were performed daily at home for six months. Two of the exercises
involved stretching and three focused on resistive strengthening.
Shoulder pain was
assessed using the “Wheelchair User’s Shoulder Pain Index” (WUSPI). With
this index, subjects reported the amount of shoulder pain associated
with 15 activities of daily living (e.g., transfers, etc.). Each
activity was assessed using a 1 (no pain) to 10 (worst pain ever
experienced) scale. The scores for all activities were combined, giving
an aggregate score ranging from 0-150.
Using this index,
subjects completing the exercise program reported a 40% reduction in
pain compared with 2% for the controls. In spite of the overall
reduction, pain levels initially increased somewhat before declining as
the exercise program was continued. Overall, the investigators
concluded that the “findings supported the effectiveness of this
exercise protocol in decreasing the intensity of shoulder pain which
interferes with functional activity in wheelchair users.”
2) In 2003, Dr A.L.
Hicks and colleagues (Canada) evaluated the impact of a long-term
exercise program on strength and a variety of other factors, including
pain, on individuals with SCI. Thirty-four individuals with traumatic
SCI at the cervical C4 level or below were recruited for the study. Age
ranged from 19 to 65 years, and the time since injury varied from one to
24 years. Eighteen and 16 subjects has tetraplegia and paraplegia,
respectively.
Of the 34 subjects,
21 were randomized into an exercise program and 13 into a control group.
Subjects in the exercise group participated in a nine-month, twice
weekly exercise program that involved both arm ergometry and resistance
training with free weights or weight machines. In contrast, control
subjects were offered a bimonthly education session on topics including
exercise physiology, osteoporosis, and relaxation techniques.
Before and after the
exercise program, subjects rated how much pain they experienced and how
it interfered with normal work over the preceding four weeks using a
six-point scale in which 1 represented “none/not at all” and 6 “very
severe/extremely.” By the end of the nine-month study period, the
exercisers reported a modest reduction in pain while the controls
reported an increase.
3) In 2006, Dr.
Deborah A. Nawoczenki and colleagues (USA) evaluated the potential
benefits of an eight-week strengthening and stretching exercise program
on existing shoulder pain in 21 manual wheelchair users with mostly SCI.
These individuals were compared to 20 asymptomatic controls. Of those in
the intervention group, age averaged 21 years, 15 were men, and the time
since injury averaged 17 years. Thirteen and eight had incomplete and
complete injuries, respectively.
The eight-week home
exercise program consisted of stretching and strengthening exercises
with elastic band resistance, focusing on specific muscles associated
with shoulder pain. The controls received no intervention. The primary
pain assessment was the previously discussed Wheelchair User’s Shoulder
Pain Index. Using this index, shoulder pain was significantly reduced
after completion of the exercise program.
4) In 2007, Dr.
Mark Nash and colleagues (USA) examined the effects of a circuit
resistance exercise training on muscle strength, endurance, anaerobic
power, and shoulder pain in seven men with paraplegia. Age ranged from
39 to 58 years, the time since injury averaged 13 years, and the injury
level varied from the thoracic T5 to T12 level. The exercise program
consisted of training three times weekly for 16 weeks with resistance
(weight lifting) and endurance (arm cranking) training. Using the
previously described Wheelchair User’s Shoulder Pain Index, shoulder
pain decreased on average from 32 before the program was started to 5 at
the end of the study.
5) In 2011, Dr.
Sara J Mulroy et al (USA) evaluated the effectiveness of an exercise
program on shoulder pain in 80 manual wheelchair users with SCI. Average
age was 45, and 71% were men. Average time since injury and duration of
shoulder pain was 20 and 5.5 years, respectively. Half of the recruited
subjects were randomized to a 12-week home-based program of shoulder
strengthening and stretching exercises together with strategies on how
to optimize transfers, raises, and wheelchair propulsion. The other half
were assigned to control group, which saw an instructional video
reviewing shoulder anatomy, mechanisms of injury, and general concepts
in managing shoulder pain.
Shoulder pain was
assessed before and after the intervention using the Wheelchair User’s
Shoulder Pain Index. Using this scale, pain levels decreased in the
exercisers from 51 to 15 after finishing the program, a decline that
persisted four weeks later. In contrast, no change was noted in the
controls.
6) In a 2012 study,
Dr. C. Norbrink and associates (Sweden) evaluated the effects of
an exercise program on both musculoskeletal and neuropathic pain
in eight individuals with SCI. Of these eight individuals, six were
males, age varied from 30-67 (average 50) years, and the time lapsing
since injury ranged from 7-29 (average 18) years. Seven and six subjects
had neuropathic and musculoskeletal pain, respectively (four had both).
One patient also had visceral pain. The level of injury ranged from the
thoracic T5 to lumbar level L1 level. The exercise program used a
double-poling ergometer adapted for persons with lower extremity
impairments. Subjects trained on this device for 50 minutes three times
a week for 10 weeks.
Before and after the
exercise program, pain was evaluated using a variety of assessments,
including the 0-10 pain scale extensively discussed elsewhere. For the
seven subjects with neuropathic pain, the average pain level decreased
from 5 to 3; and two reported much improvement, two minimal improvement,
and three no change. For those individuals with musculoskeletal pain,
average pain intensity declined from 4 at baseline to 0 at study end.
All but one had no musculoskeletal pain at the end of the study, and the
number of days per week with pain declined from an average of 5.5 to 0.7
days.
Five of the eight
subjects had reported shoulder pain at the beginning of the study. In
these individuals, shoulder pain was also measured before and after the
program using the Wheelchair User’s Shoulder Pain Index. Using this
0-150 assessment, shoulder pain decreased from an average of 37 to 18
after the program was completed.
The investigators
noted that the impact of the exercise program on neuropathic pain
appears to be comparable to many of the pharmaceutical drugs studied for
treating neuropathic pain. They concluded “Despite the lack of studies
on exercise as a treatment for SCI neuropathic pain, we recommend
health-care staff to consider prescribing regular physical training for
this group as it is a safe treatment option with many more advantages
than just pain relief.”
TOP |
| |
| |
|
| |
| |
|
| |
| |
|
|